-
Is work energy?
NO
-
Work is not actually a form of energy itself, but a _____.
process by which energy is transferred from one system to another
-
Work and _____ are the only two ways to transfer energy from one system to another.
heat
-
Work SI units
Joule (J)
-
The transfer of energy by work or heat is the _____.
only way by which anything occurs
-
The potential energy in the ATP is harnessed by _____.
heat transfer
-
At the molecular level, work and heat are _____.
effectively the same.
-
In real life, work is _____, and energy is usually lost as _____.
never perfect
thermal energy
-
Work formula (mechanics)
W = F · d = Fd cos θ
...where W is work, F is the magnitude of the applied force, d is the magnitude of the displacement through which the force is applied, and θ is the angle between the applied force vector and the displacement vector.
-
only forces _____ to the displacement vector will do work.
parallel or antiparallel
-
In systems of gasses, work can be thought of as a combination of _____.
pressure and volume changes
-
In fluids, pressure can be though of as an "_____".
energy density
-
We say that work has been done on a piston when the volume of the system has _____.
changed due to an applied pressure
-
Gas expansion and compression processes can be represented in graphical form with volume on the _____ and pressure on the _____. These are called _____.
x-axis
y-axis
P–V graphs
-
The work done on or by a system undergoing a thermodynamic process can be determined by finding the _____ enclosed by the corresponding pressure–volume curve.
area
-
When a gas expands, we say that work was done _____ and the work is _____; when a gas is compressed, we say that work was done _____ and the work is _____.
by the gas, positive
on the gas, negative
-
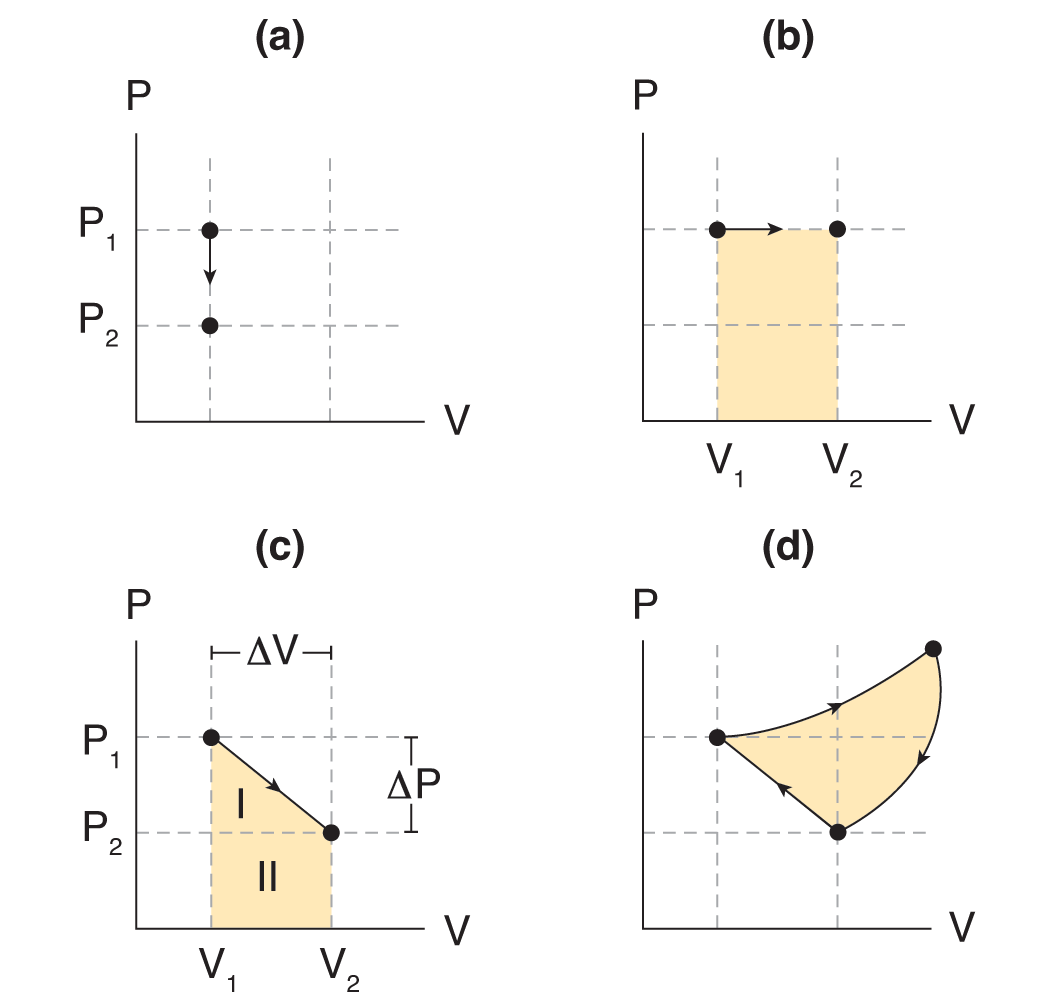
Is work done on a gas path-independent?
No (see P-V curves)
-
If volume stays constant as pressure changes (that is, ΔV = 0), then _____.
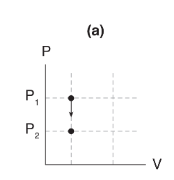
no work is done
-
Processes where volume stays constant (ΔV = 0) are called _____ or _____ processes.
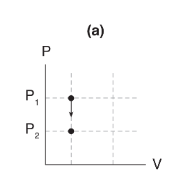
isovolumetric or isochoric process.
-
If pressure remains constant as volume changes (that is, ΔP = 0), then work is _____.
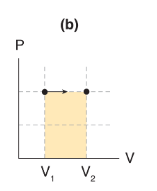
the area under the curve, which is a rectangle of length P and width ΔV
-
Processes where pressure stays constant (ΔP = 0) are called _____ processes.
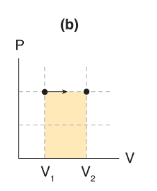
isobaric
-
Isobaric work formula
W = PΔV
-
Straight line work formulas for a piston (linear change in pressure and volume)
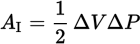
AII = P2ΔV
W = AI + AII
-
Power refers to the rate at which _____.
energy is transferred from one system to another
-
SI derived unit of power
watt (W)
(equal to J/s)
-
Power equation
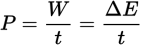
-
Many of the devices we use every day—toaster ovens, light bulbs, phones, cars, and so on—are quantified by the rate at which these appliances _____.
transform electrical potential energy into other forms
-
Power is always a measure of the rate of _____ per _____.
energy consumption, transfer, or transformation
unit time
-
work–energy theorem for mechanics (equation)
Wnet = ΔK = Kf – Ki
-
work–energy theorem for mechanics (definition)
The net work done by forces acting on an object will result in an equal change in the object’s kinetic energy.
-
What is the implication of the work-energy theorem in mechanics (specifically on the MCAT)?
If one calculates the change in kinetic energy experienced by an object, then—by definition—the net work done on or by an object is the same.
-
The first law of thermodynamics is essentially a _____, in which the change in _____ is equal to the _____ minus the _____.
reiteration of the work–energy theorem
internal energy (ΔU)
heat transferred into the system (Q)
mechanical work done by the system (W)
ΔU=Q-W

