-
removal of dimethylamino group
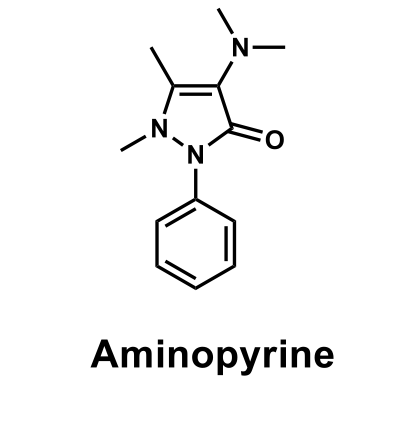
What biosteric modification changed aminopyrine's carcinogenic effect; resulting in Propylphenazone?
-
Bioisosteres
-substituents or groups with similar physical or chemical properties which produce broadly similar biological properties in a chemical compound.
-
monovalent, divalent, trivalent, tetrasubstituted atoms, ring equivalents
What groups belong to classical bioisosteres?
-
cyclic and noncyclic isosteres, exchangeable groups
What groups belong to nonclassical bioisosteres?
-
a,d
What element is an isostere of H?
a) Fluorine
b) Oxygen
c) Magnesium
d) Deuterium
-
to block metabolically liable site in hopes that it does not impair binding to target
Why do we use F as an Isostere?
-
d
What is the most conservative example of biosterism in regards to H atom?
a) Fluorine
b) Oxygen
c) Magnesium
d) Deuterium
-
lowers pKa; more acidic
What effect does fluorine have on pKa?
-
can redirect metabolic pathways
How does replacing Hydrogen with Deuterium affect metabolism?
-
C-D
Which bond is more stable?
C-H or C-D
-
decreases CYP2D6 activity; causing this compound to be metabolized faster; less chance for ddi
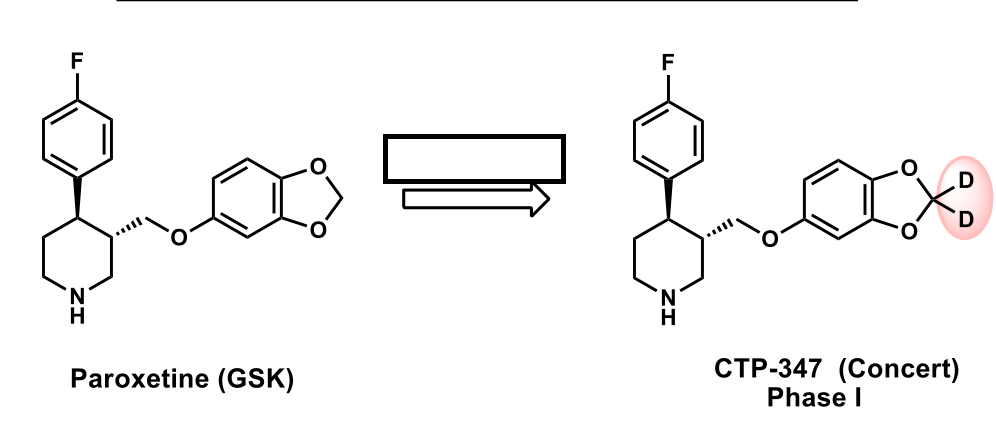
How did Deuterium resolve some of the implications associated with Paroxetine?
-
4; it is acidic due to it being next to ketones
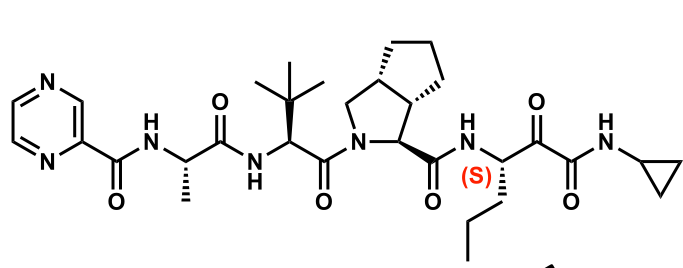
How many chiral centers are in this compound? Why does S get racemized and not the other chiral centers?
-
slows epimerization
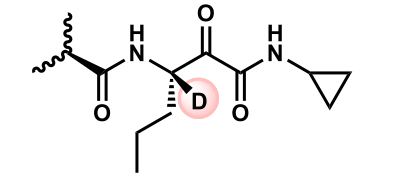
How does adding deuterium affect this compounds metabolism?
-
huntington's disease; adding d prevents o-demethylation increasing drug half-life
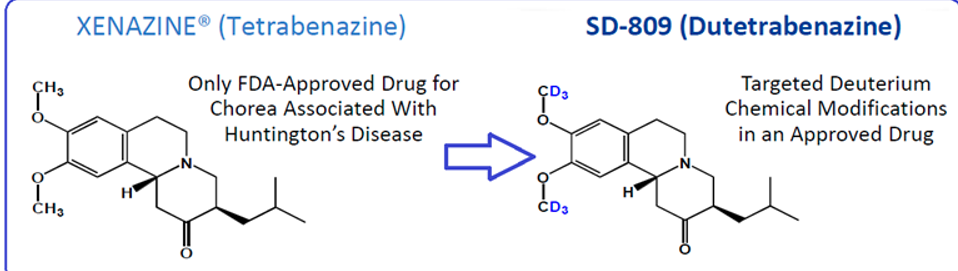
What disorders are these drugs used to treat? How does adding deuterium improve this drug?
-
Tetrazine; carboxylic acid
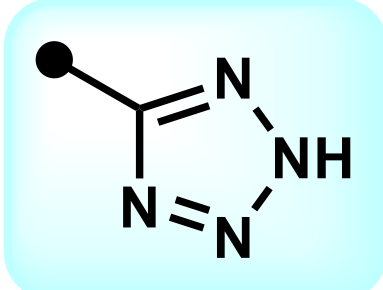
What is the name of this functional group? What isostere group does it belong to?
-
D & F
What are isosteres of H?
-
sulfonamide; carboxylic acid
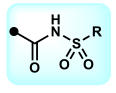
What is the name of this functional group? What isostere group does it belong to?
-
carbamate; carboxylic acid
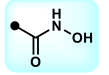
What is the name of this functional group? What isostere group does it belong to?
-
increase binding affinity by adding groups with more HBA; tetrazine
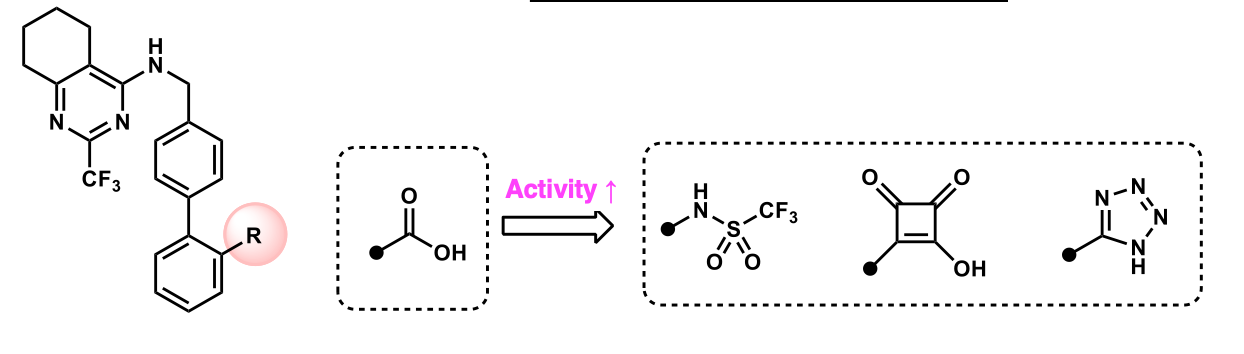
What is the goal of replacing carboxylic acid with the following isosteres? Which isostere gives us the best binding affect?
-
Nonclassical bioisosteres
-structurally distinct, usually comprised of different atoms and exhibit different steric and electronic properties.
-
prevented thymidylate synthase from metabolizing the drugs
How did the addition of Fluorine in 5-Fluorouracil & Trifluridine improve it?
-
increase binding by replacing cooh with groups that have more hba's
What are uses of carboxylic acid isosteres?
-
increases pka; more acidic and faster clearance
What does Fluorine offer as an isostere?

