Introduction to Clinical Renal Medicine (Physiology)
Session summary Why it is important: This lecture gives an overview of clinical aspects of major forms of renal disease including acute kidney injury, chronic kidney disease and nephrotic syndrome. It illustrates how knowledge of the basic sciences can help understanding the consequences of renal disease. Learning outcomes At the end of this session you will be able to: Understand the scope of renal diseases and how patients can present with kidney problems Understand the importance of renal physiology in clinical renal medicine Gain insights into how advances in physiological understanding have enabled the development of treatments for kidney disease
-
What are the main functions of the kidneys? (6)
The main functions of the kidneys include:
𖦹 Excreting water-soluble waste
𖦹 Controlling blood pressure
𖦹 Maintaining electrolyte balance
𖦹 Regulating water balance
𖦹 Ensuring acid-base homeostasis
𖦹 Serving endocrine functions, such as producing renin-angiotensin-aldosterone system components, erythropoietin, and activating vitamin D.
-
What are the main areas covered in renal medicine? (7)
The scope of renal medicine includes:
𖦹 Acute kidney injury (AKI)
𖦹 Chronic kidney disease (CKD)
𖦹 Renal replacement therapies
𖦹 Glomerular diseases
𖦹 Tubulointerstitial diseases
𖦹 Acid/base disturbances
𖦹 Electrolyte disturbances
-
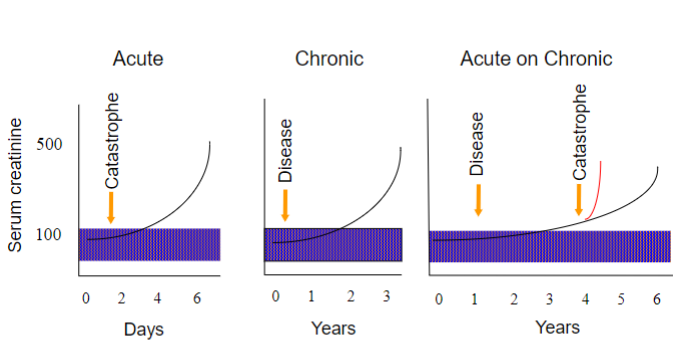
Picture demonstrating the tempo of presentation:
-
What is the median life expectancy on renal replacement therapy (RRT) after 90 days, categorized by age group for incident patients starting RRT from 2000-2009? (No harsh marking)
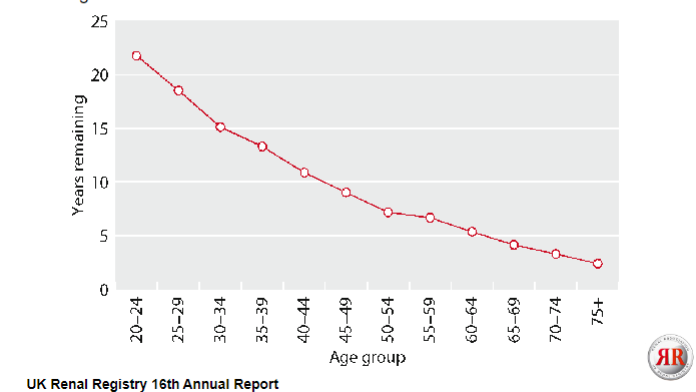
The median life expectancy on RRT after 90 days, categorized by age group for incident patients starting RRT from 2000-2009 varies based on age:
For patients aged 0-14 years: approximately 22.6 years
For patients aged 15-24 years: approximately 30.3 years
For patients aged 25-34 years: approximately 32.2 years
For patients aged 35-44 years: approximately 29.6 years
For patients aged 45-54 years: approximately 25.7 years
For patients aged 55-64 years: approximately 20.1 years
For patients aged 65-74 years: approximately 15.4 years
For patients aged 75-84 years: approximately 9.0 years
For patients aged 85 years and older: approximately 3.6 years
-
How is kidney function typically measured? (1)
𖦹 Kidney function is typically measured by the volume of fluid filtered from the glomerular capillaries in a specified period of time.
-
What are the typical values for glomerular filtration rate (GFR) in young males and females? (2)
𖦹 In young males, the typical GFR is approximately 130 ml/min/1.73 m^2
𖦹 While in young females, it is approximately 120 ml/min/1.73 m^2.
-
How does GFR change with age? (1)
𖦹 GFR remains stable until around age 40 and then declines at a rate of approximately 1 ml/min/1.73 m^2 per year.
-
What is the approximate GFR at age 80 compared to a young adult? (1)
𖦹 At age 80, the mean GFR is approximately half that of a young adult.
-
What are some methods used to measure kidney function? (3)
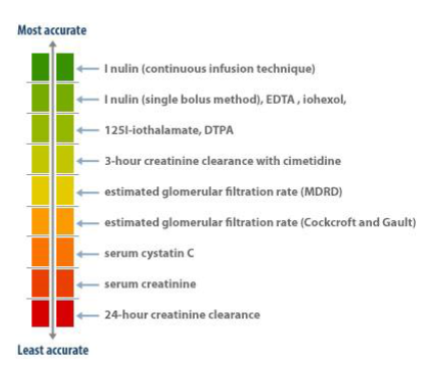
𖦹 Blood tests (such as creatinine levels and formulae)
𖦹 Measurement of urine output
𖦹 Assessment of the elimination of radioisotopes
-
How is glomerular filtration rate (GFR) measured in the real world? (4)
𖦹 GFR is commonly estimated using markers such as creatinine and urea.
𖦹 Creatinine, derived from muscle cells, is the closest to an ideal endogenous marker.
𖦹 Its steady production results in a steady state in plasma, and small changes in creatinine levels reflect large changes in GFR.
𖦹 Urea, though also used, is considered less reliable as its levels are vulnerable to changes for various reasons.
-
How is glomerular filtration rate (GFR) calculated? (3)
𖦹 GFR can be estimated using formulas that incorporate factors like serum creatinine levels.
𖦹 One commonly used formula is the CKD-EPI equation, which calculates GFR using the following formula: GFR = 141 × min(Scr/κ, 1)^α × max(Scr/κ, 1)^-1.209 × 0.993^Age × 1.018 [if female] Where:
Scr is serum creatinine (mg/dL)
κ is 0.7 for females and 0.9 for males
α is –0.329 for females and –0.411 for males
min indicates the minimum of Scr/κ or 1
max indicates the maximum of Scr/κ or 1
-
Picture demonstrating a decline in GFR as we age:
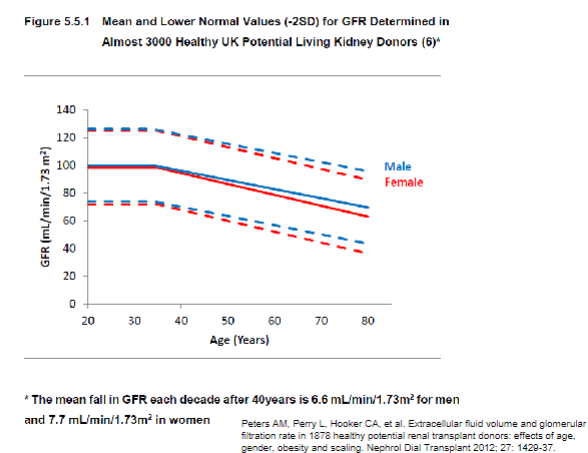
-
Picture demonstrating a decline in GFR as we age at the nephrons:
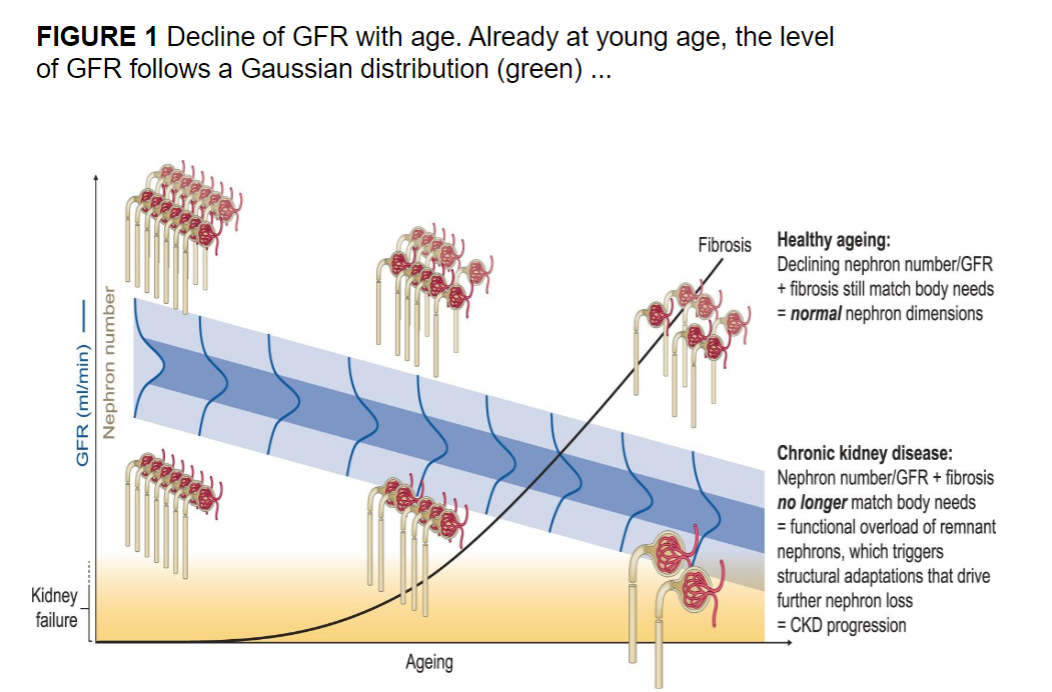
-
Picture of Case 1 AKI (Actue Kidney Injury):

-
Picture demonstrating Mortality & change in serum creatinine:
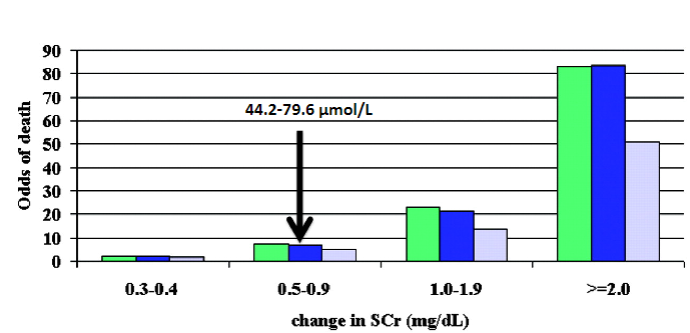
-
Picture demonstrating Standardising diagnosis - KDIGO:
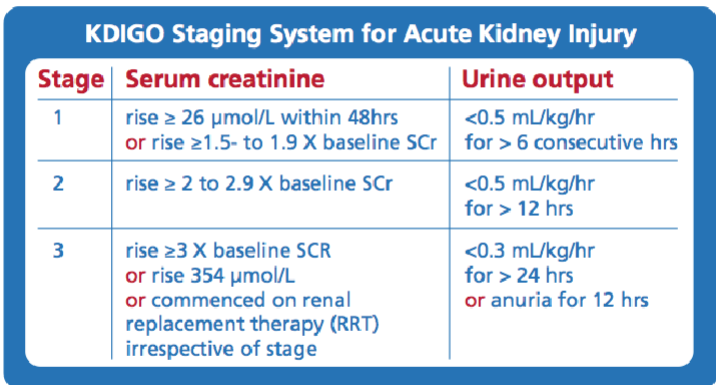
-
Picture demonstrating if electronic alerts improve outcomes in patients with acute kidney injury: (Answer is NO)
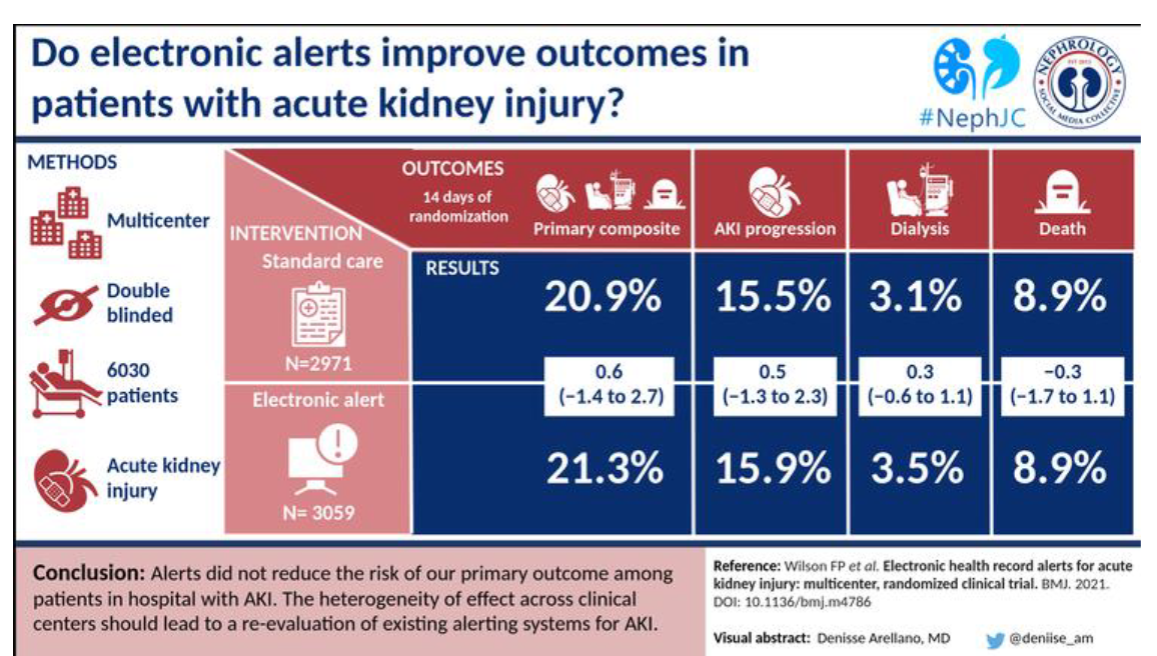
-
Picture demonstrating AKI stages and mortality:
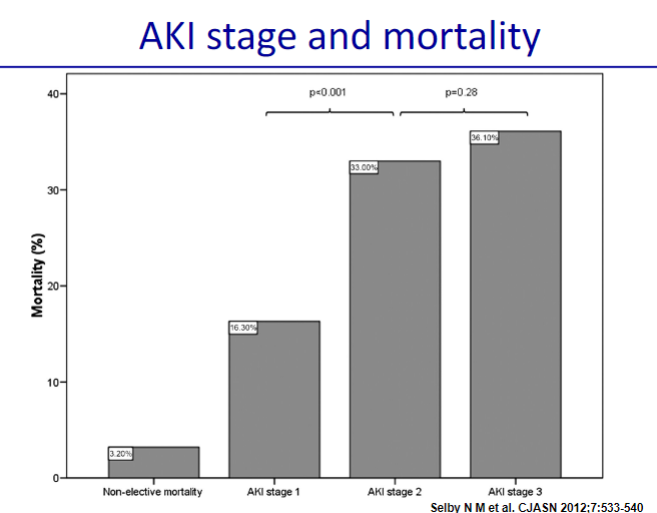
-
Picture demonstrating the phases of AKI:
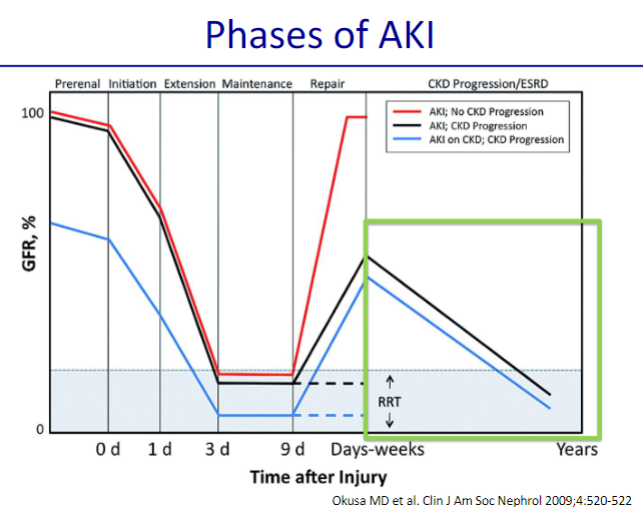
-
Picture demonstrating the causes of AKI:
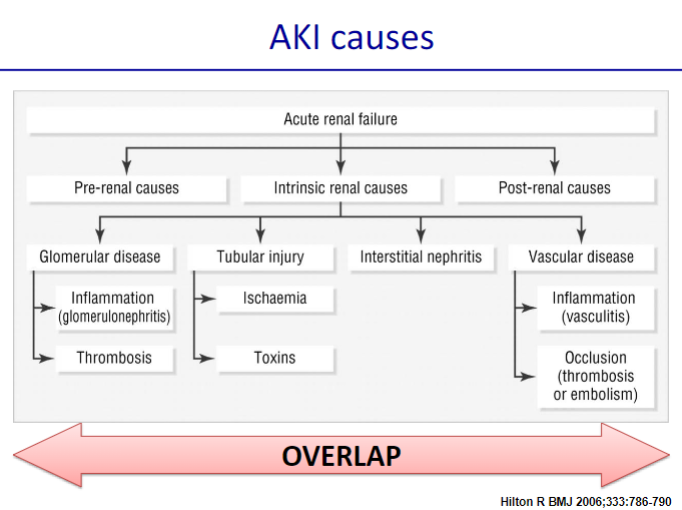
-
What causes pre-renal acute kidney injury related to decreased kidney perfusion? (11)
𖦹 Hypovolemia
𖦹 Hemorrhage
𖦹 Volume depletion
𖦹 Hypotension
𖦹 Cardiogenic shock
𖦹 Septic shock
𖦹 Renal hypoperfusion/vasoconstriction
𖦹 NSAIDs (Non-Steroid Anti-inflammatory Drugs)
𖦹 ACE inhibitors or ARBs
𖦹 Abdominal aortic aneurysm (AAA), renal artery stenosis (RAS), or occlusion
𖦹 Hepatorenal syndrome
-
Picture demonstrating intrinsic renal disease:
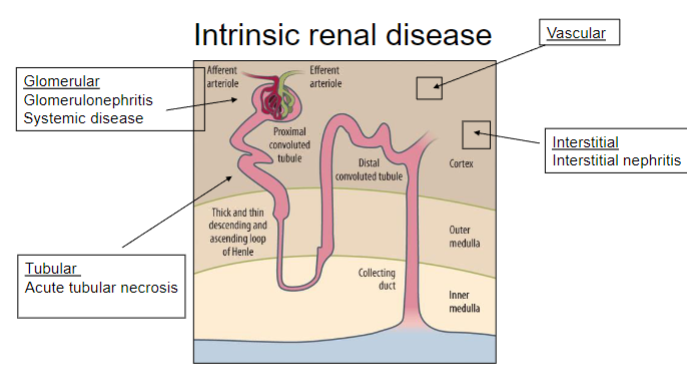
-
What are the implications of obstructive uropathy in acute kidney injury? (4)
𖦹 Prostatic obstruction causes 25% of AKI
𖦹 Single remaining kidneys at high risk
𖦹 Can still produce significant amounts of urine
𖦹 Delay in correction (catheter or nephrostomy) compromises renal function permanently
-
Picture demonstrating when RRT (Renal Replacement Therapy) should be started:
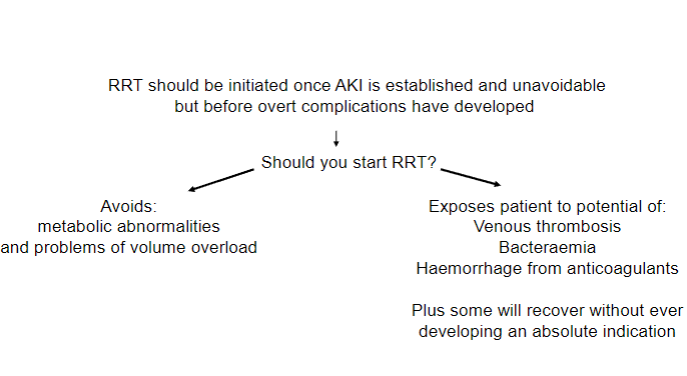
-
Indications for acute dialysis (4)
𖦹 Hyperkalaemia refractory to medical therapy: K+ > 6.5 (mmol/L) with ECG changes.
𖦹 Severe Acidosis: pH < 7.25, HCO3 <15.
𖦹 Fluid overload: despite high-dose furosemide appropriate.
𖦹 Symptomatic uraemia: urea > 35 (mmol/L), leading to conditions such as pericarditis and encephalopathy.
-
What is hyperkalaemia? (1)
𖦹 Hyperkalemia refers to elevated levels of potassium in the blood.
-
What is the scheme for approaching glomerular disease? (4)
• Presentation of the patient
• Pathogenesis (often poorly understood)
• Histopathology
• Together these factors help us plan the treatment course and optimise patient outcomes
-
What are some presentations of glomerulonephritis? (5)
• Asymptomatic urinary abnormalities
• CKD
• Nephrotic syndrome
• Nephritic syndrome
• Rapidly progressive GN (Glomerulonephritis)
-
How is proteinuria quantified in urine analysis for glomerular diseases? (2)
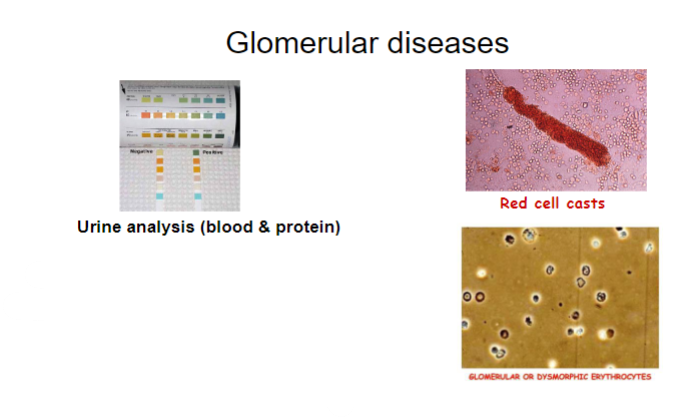
Proteinuria quantified by:
•urine albumin:creatinine ratio
•urine protein:creatinine ratio
-
What is proteinuria? (1)
𖦹 Proteinuria is the presence of an abnormal amount of protein in the urine.
-
What are the diagnostic criteria for nephrotic syndrome? (3)
Nephrotic syndrome
• Albumin <30 g/L
• Proteinuria >3g/24 hours
• +/- oedema
-
What is the major cause of Chronic Kidney Disease? (1)
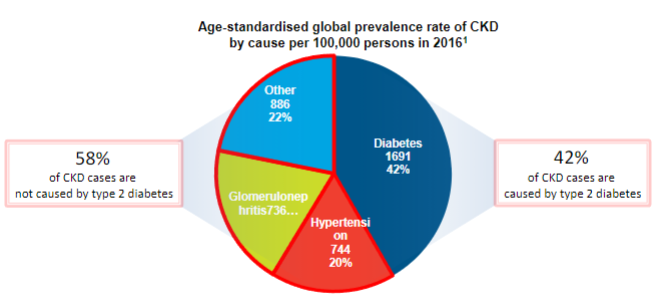
Type 2 diabetes
-
Who should be tested for chronic kidney disease (CKD)? (12)
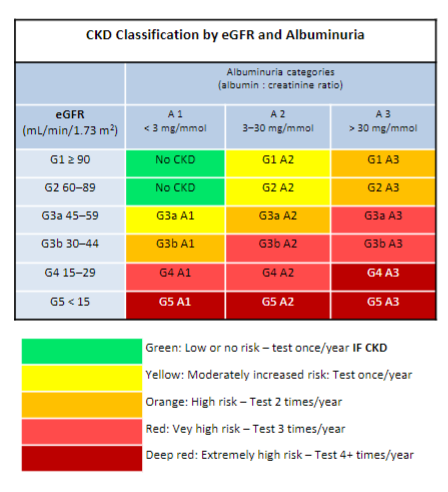
Patients with the following:
𖦹 Diabetes
𖦹 Hypertension
𖦹 History of acute kidney injury (monitor for 3 years even if function back to baseline)
𖦹 Cardiovascular disease (ischaemic heart disease, chronic heart failure, peripheral vascular disease or cerebral vascular disease)
𖦹 Structural renal tract disease, recurrent renal calculi or prostatic hypertrophy
𖦹 Multisystem disease, e.g., systemic lupus erythematosus
𖦹 Family history of end-stage kidney disease (GFR category G5) or hereditary kidney disease
𖦹 Gout
𖦹 Incidental detection of haematuria or proteinuria
𖦹 On nephrotoxic agents such as, lithium, calcineurin inhibitors, sulphasalazine, long term chronic use of NSAIDS
𖦹 Solitary functioning kidney
-
Risk factors for GFR decline (10)
𖦹 Obesity
𖦹 Diabetes
𖦹 Hypertension
𖦹 Cardiovascular disease
𖦹 AKI
𖦹 Proteinuria
𖦹 Smoking
𖦹 NSAIDs
𖦹 Ethnicity
𖦹 Untreated urinary outflow tract obstruction
-
Picture demonstrating the classical course of diabetic kidney disease:
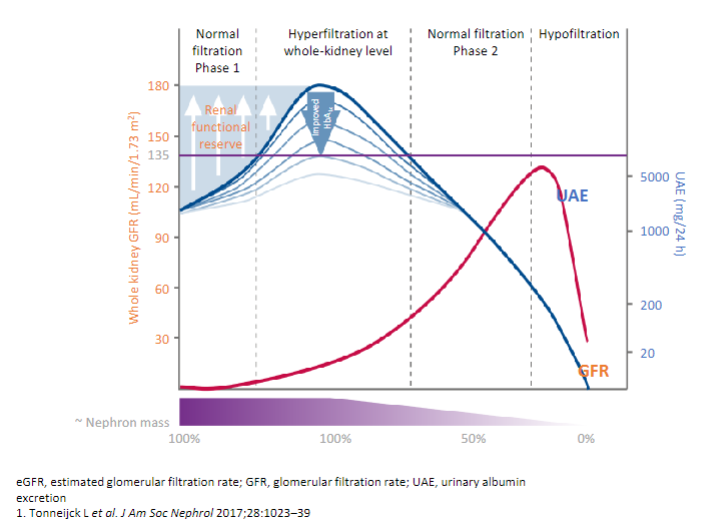
-
Picture demonstrating the RAAS (Renin-Angiotensin-Alsosterone -System)
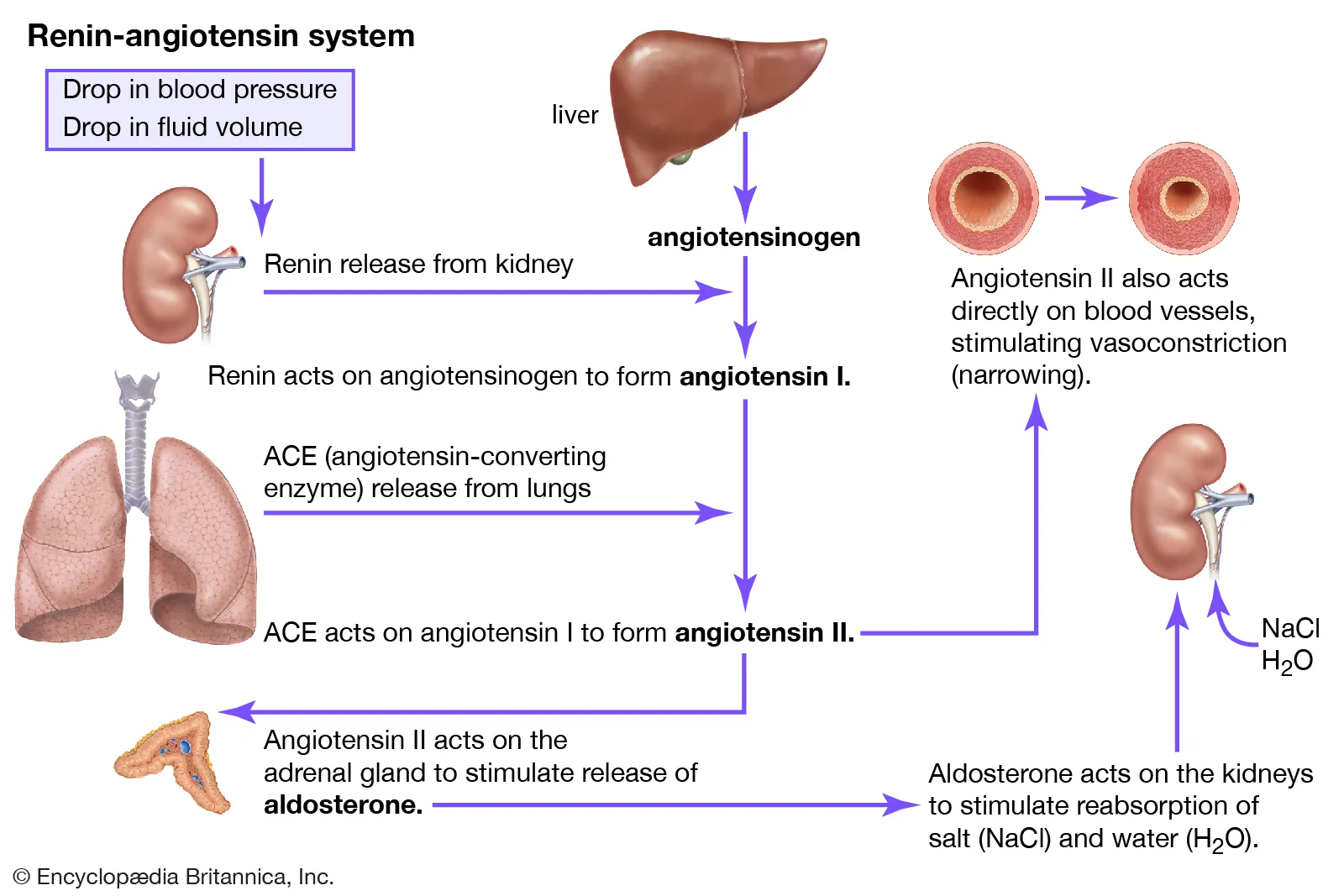
-
Picture demonstrating a healthy nephron vs a diabetic nephron:
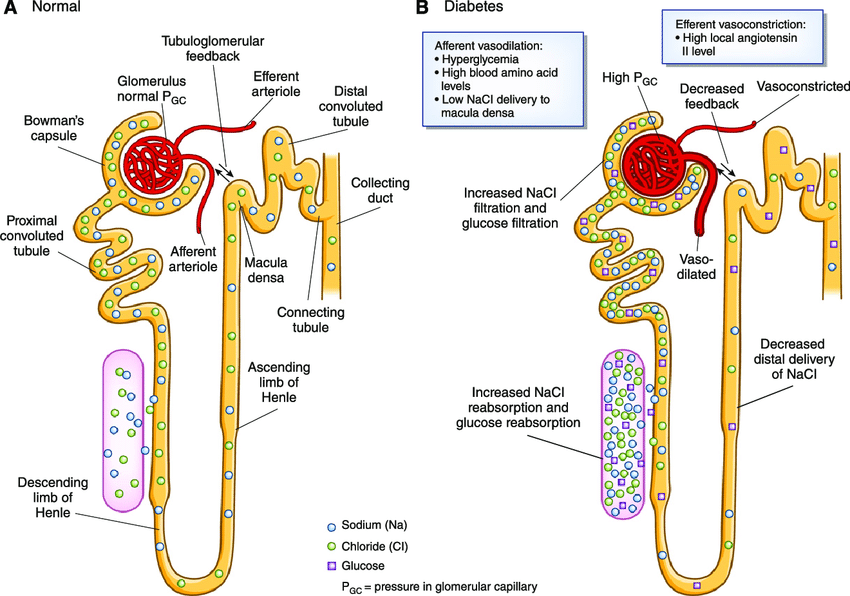
-
How do SGLT2 inhibitors affect chronic kidney disease (CKD)? (5)
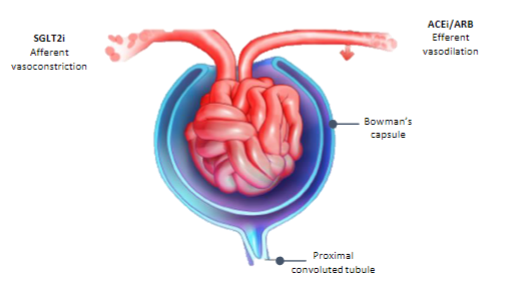
𖦹 Inhibiting SGLT2 reduces glucose reabsorption from the glomerular filtrate in the proximal renal tubule.
𖦹 This reduction in glucose reabsorption leads to urinary excretion of glucose and osmotic diuresis.
𖦹 SGLT2 inhibitors also decrease sodium reabsorption, which increases sodium delivery to the distal tubule.
𖦹 Increased sodium delivery to the distal tubule is thought to enhance tubuloglomerular feedback and reduce intraglomerular pressure.
𖦹 The preservation of kidney function by SGLT2 inhibitors is not solely due to their blood glucose-lowering effect and is not limited to patients with type 2 diabetes.
-
What are some complications associated with declining glomerular filtration rate (GFR) and end-stage renal failure (ESRF)? (4)
𖦹 Haematological: Anemia due to decreased production of erythropoietin by the kidneys, leading to reduced red blood cell production.
𖦹 Bone: Renal osteodystrophy, a condition characterized by bone mineral density loss, skeletal deformities, and an increased risk of fractures.
𖦹 Cardiovascular (CVS): Increased risk of cardiovascular diseases such as hypertension, heart failure, and coronary artery disease due to fluid overload, electrolyte imbalances, and uremic toxins.
𖦹 Other: Immunological dysfunction, including impaired immune response and increased susceptibility to infections. Malnutrition due to poor appetite, dietary restrictions, and metabolic abnormalities commonly seen in advanced kidney disease.
-
What is acidemia? (1)
Acidemia is a medical condition characterized by an abnormally low blood pH, typically below the normal range of 7.35 to 7.45
-
How can acidemia be corrected? (5)
𖦹 Treating the underlying cause: Identifying and addressing the condition responsible for acidemia is crucial. For example, treating respiratory disorders causing hypoventilation or metabolic disorders causing excess acid production.
𖦹 Intravenous fluids: Administration of intravenous fluids containing bicarbonate can help correct acidemia by buffering excess acids in the blood.
𖦹 Medications: Depending on the cause, medications such as bronchodilators for respiratory conditions or insulin for diabetic ketoacidosis may be necessary.
𖦹 Dialysis: In cases of severe acidemia due to kidney failure (uremia), dialysis may be required to remove accumulated acids and restore normal blood pH.
𖦹 Monitoring: Close monitoring of blood pH levels and electrolyte balance is essential during treatment to ensure correction of acidemia without causing complications.
-
What do the kidneys convert? (1) And a dysfunction in this conversion leads to what? (1)
𖦹 Kidneys 1 α hydroxylate vit D3: The kidneys play a crucial role in activating vitamin D3 by converting it to its active form, 1,25-dihydroxyvitamin D3.
𖦹 Bone mineral disease occurs: Dysfunction in this process can lead to disturbances in calcium and phosphate metabolism, contributing to bone mineral disease.
-
How does kidney dysfunction contribute to bone mineral disease? (6)
↓ Glomerular filtration rate: Decreased filtration leads to impaired clearance of phosphate.
↓ Filtered phosphate load: Reduced phosphate clearance results in increased phosphate retention.
Phosphate retention: Elevated phosphate levels contribute to bone demineralization.
↓ 1,25 (OH)2 Vit D3 synthesis → ↓calcium: Decreased activation of vitamin D leads to reduced intestinal calcium absorption.
Directly increases PTH: Elevated phosphate and decreased calcium levels stimulate parathyroid hormone (PTH) secretion.
PTH release: PTH acts on bone and kidneys to mobilize calcium and increase phosphate excretion.
Long term leads to secondary hyperparathyroidism & bone disease:
-
Picture demonstrating therapy of CKD-MBD in dialysis
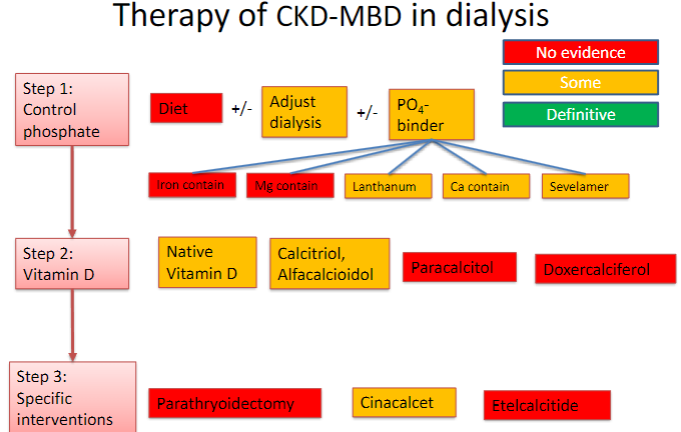
-
What is anemia, and how does it relate to chronic kidney disease (CKD)? (7)
Definition: Anemia is characterized by a reduced number of circulating red blood cells.
Prevalence: It is common and debilitating, affecting approximately 80% of patients with advanced renal impairment.
Key Indicator: Hemoglobin (Hb) levels serve as a primary indicator of anemia.
CKD Rates: The prevalence of anemia varies with CKD stage:
68% of patients on renal replacement therapy (RRT) experience anemia.
In CKD Stage 3, approximately 5.2% of patients have anemia.
This figure increases to 44% in CKD Stage 4.
-
Picture demonstrating the mechanisms of renal anaemia:
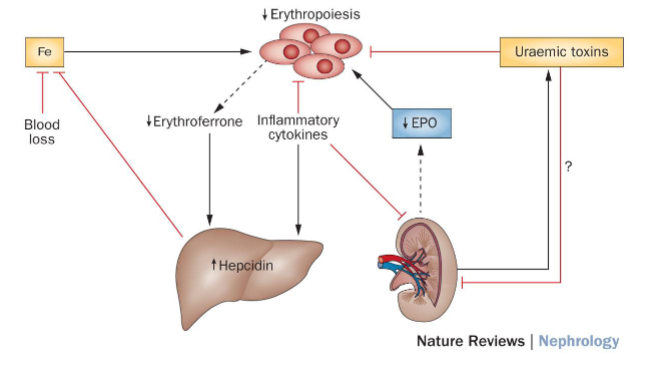
-
How does HIF (Hypoxia-Inducible Factor) coordinate erythropoietin production with iron metabolism? (4)
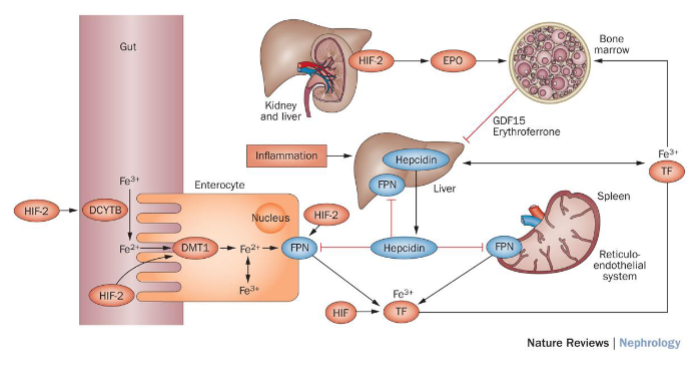
HIF Function: HIF is a transcription factor that regulates gene expression in response to changes in oxygen levels.
Erythropoietin (EPO) Production: In hypoxic conditions (low oxygen levels), HIF promotes the expression of the erythropoietin gene (EPO), leading to increased production of EPO.
Iron Metabolism: HIF also regulates genes involved in iron metabolism, including those responsible for iron absorption, transport, and utilization.
Synergistic Regulation: By coordinating EPO production with iron metabolism, HIF ensures that adequate iron is available for hemoglobin synthesis in response to increased erythropoiesis.
-
What is ESRF (Definition, kidney functions, key roles)? (3)
𖦹 Definition: Renal failure necessitating renal replacement therapy.
𖦹 Kidney Functions: Toxin elimination, fluid and electrolyte balance, acid-base equilibrium, hormonal regulation.
𖦹 Key Roles: Vitamin D activation, erythropoietin production, metabolic functions.
-
What medical therapies are utilized in the treatment of renal failure? (7)
𖦹 Anaemia management
𖦹 Blood pressure control
𖦹 Diuresis promotion
𖦹 Bicarbonate supplementation
𖦹 Phosphate control
𖦹 Secondary hyperparathyroidism management
𖦹 End-stage renal failure management
-
What does maximum conservative care refer to? (3)
𖦹 Non-dialytic management of end-stage renal disease (ESRD)
𖦹 Focuses on optimizing medical therapies to manage symptoms and slow disease progression
𖦹 Includes strategies such as managing anaemia, controlling blood pressure, addressing fluid balance, and treating metabolic abnormalities
-
When is the initiation of Renal Replacement Therapy (RRT) considered? (4)
𖦹 It's ideal not to delay initiation until the onset of life-threatening complications.
𖦹 Symptoms can be subjectively interpreted; some patients accommodate to them, and medications can mimic symptoms.
𖦹 Quantitative measurements like the estimation of Glomerular Filtration Rate (GFR) are used.
𖦹 MDRD and Cockcroft-Gault equations may overestimate GFR in CKD stages 4 and 5.
-
Picture demonstrating the process of renal replacement therapy:
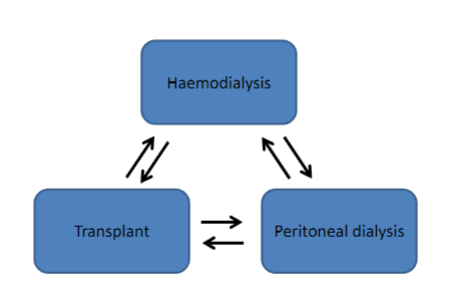
-
What are the mechanisms of solute movement across membranes?
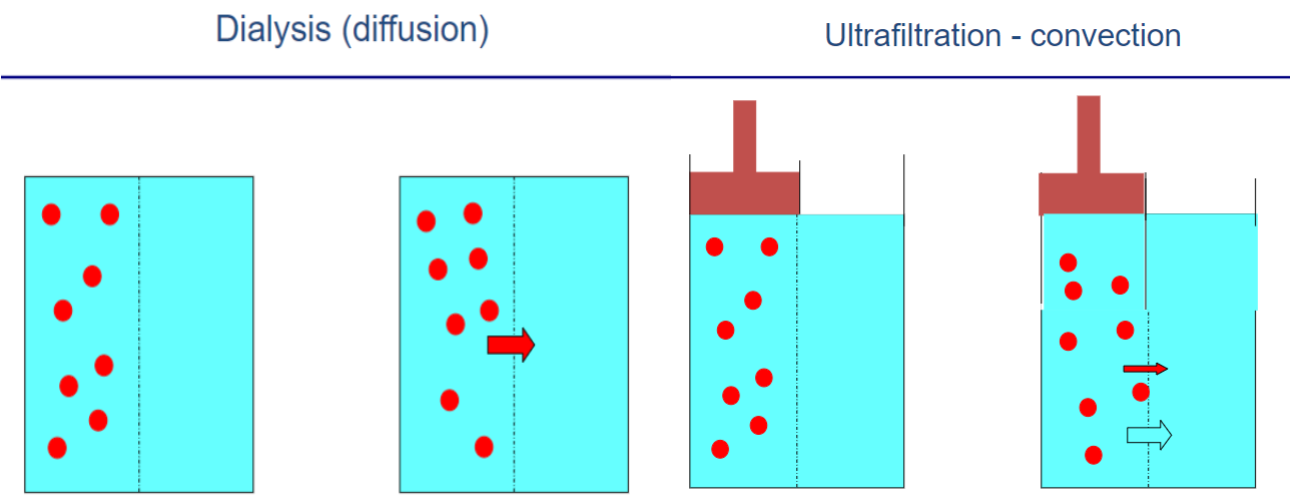
𖦹 Diffusion: Occurs when there is a difference in solute concentration on either side of the membrane.
𖦹 Convection: The movement of solute in association with water, also known as solute drag.
-
Who developed the first human hemodialysis machine, and when was it first used on humans? (3)

𖦹 Georg Haas (1886 - 1971)
𖦹 The Haas dialyzer, developed in 1923, was first used on humans in 1924 in Giessen.
𖦹 Georg Haas treated an acute patient in Giessen in 1926.
-
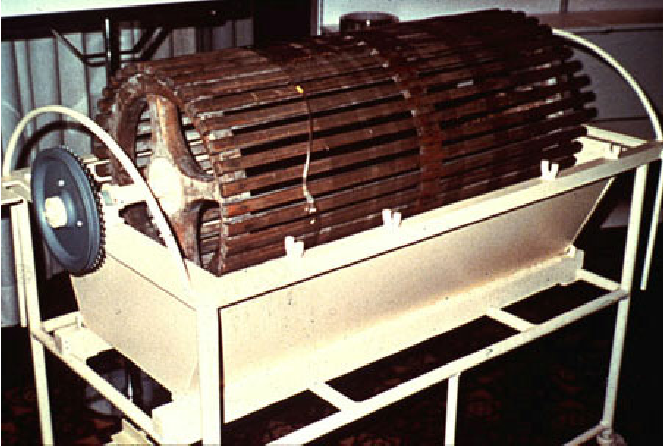
Who is considered the pioneer of hemodialysis, and when was his first rotating artificial kidney used for dialysis? (2)

𖦹 Willem Kolff (1911-2009)
𖦹 Kolff's first rotating artificial kidney was used for dialysis.
-
What are the basic principles of hemodialysis? (3)
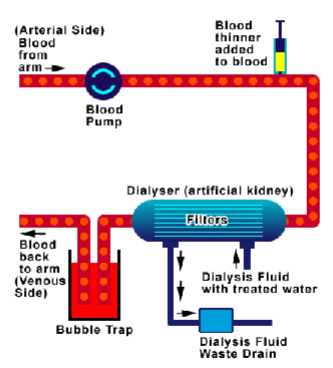
𖦹 Blood passes down one side of a highly permeable membrane.
𖦹 Water and solutes pass across the membrane, including solutes up to 20,000 daltons, such as drugs and electrolytes.
𖦹 Replacement solution with physiologic concentrations of electrolytes is infused.
-
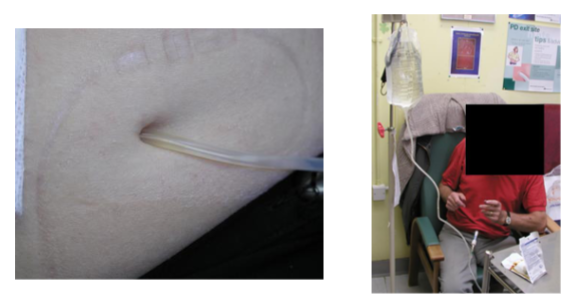
What is peritoneal dialysis? (3)
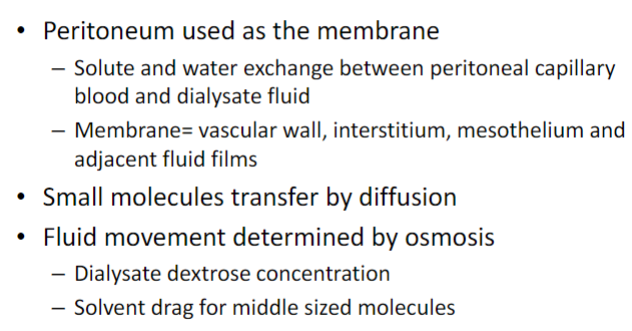
𖦹 Peritoneal dialysis is a type of dialysis that uses the peritoneal membrane in the abdomen as a semipermeable membrane for fluid and solute exchange.
𖦹 A cleansing solution (dialysate) is infused into the abdomen through a catheter, and waste products and excess fluids pass from the blood vessels in the peritoneal membrane into the dialysate.
𖦹 After a dwell time, the used dialysate is drained from the abdomen, taking waste products with it.
-
What are the types of kidney donors in transplantation? (5)
Live donors
𖦹 Related
𖦹 Unrelated
𖦹 Altruistic
Deceased donors
𖦹 Donation after brain death (DBD)
𖦹 Donation after circulatory death (DCD)
-
What are the key aspects of renal medicine? (5)
𖦹 Measuring renal function
𖦹 Importance of urinary findings
𖦹 Understanding the tempo of the disease
𖦹 General and specific treatments for conditions
𖦹 Managing the patient journey from the onset of kidney problems to kidney failure and beyond

