Introduction to Clinical Neuroscience (Physiology 2) UNFINISHED
Introduction to Clinical Neuroscience - Prof Franklyn Howe Lecture Outline Neuroimaging is key to the diagnosis for a wide range of diseases and for understanding brain development and function. A brief description of how CT, MRI and PET work will be given along with their application to various diseases. Desired Learning Outcomes Describe the brain in terms of cortical grey and white matter and their cellular structure. Understand that the brain has localised functionality (sensory/motor homunculus) and distributed connectivity (white matter pathways) Describe the signal source that is used to create CT, MRI and PET images Understand that pathological processes lead to altered tissue structure and altered appearance on images List the types of information that can be obtained from CT, MRI and PET to aid diagnosis Session Resources Introduction to Clinical Neuroscience Phys II - Part I.pdfDownload Introduction to Clinical Neuroscience Phys II - Part I.pdf Minimise file preview Introduction to Clinical Neuroscience Phys II - Part II.pdfDownload Introduction to Clinical Neuroscience Phys II - Part II.pdf Introduction to Clinical Neuroscience Phys II - Part III.pdfDownload Introduction to Clinical Neuroscience Phys II - Part III.pdf Introduction to Clinical Neuroscience Phys II - Summary and answers.pdfDownload Introduction to Clinical Neuroscience Phys II - Summary and answers.pdf Session Activities Introduction to Clinical Neuroscience - Part I Part II Part III Additional Resources Neuroscience: Exploring the Brain 3rd or 4th Edition (Bear, Connors, Paradiso). Glossary CT – computer tomography. Method of 2D and 3D imaging be measuring X-ray attenuation through tissue MRI – magnetic resonance imaging. 2D and 3D imaging by detecting the signal from the protons in water and fat molecules. A wide variety of imaging methods are possible including T1, T2, diffusion, perfusion, and functional. T2-weighted MRI. An MRI method which produces images with the signal intensity related to the tissue T2 relaxation time. T2 increases with increased water content T1-weighted MRI. An MRI method which produces images with the signal intensity related to the tissue T1 relaxation time. T1 is different between grey and white due to their different cellular structure. Diffusion-weighted MRI. An MRI method that allows the microscopic diffusion of water molecules to be measured in a specific direction. PET – positron emission tomography. 2D imaging by detecting the gamma rays produced from an injected radiopharmaceutical Radiopharmaceutical – a molecule that can be given intravenously or orally that has a radioisotope attached and that has diagnostic or therapeutic use. The molecule may have properties that allow it to target specific cell receptors, organs, or undergo specific metabolic processes in the body.
-
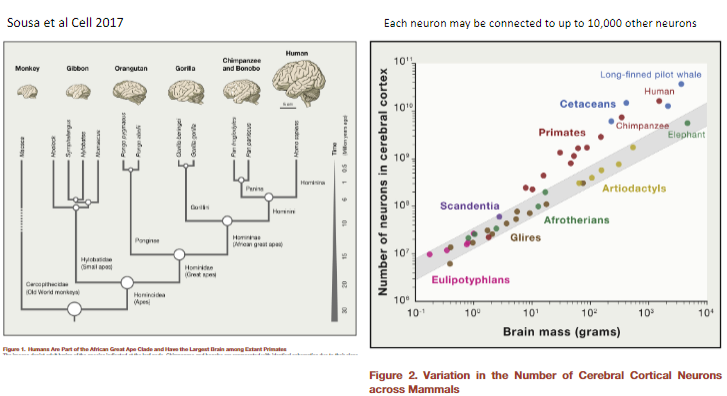
Picture demonstrating the evolution of the humans brain:
-
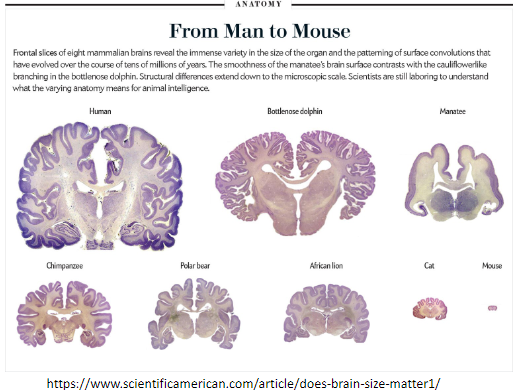
Picture demonstrating from man to mouse:
-
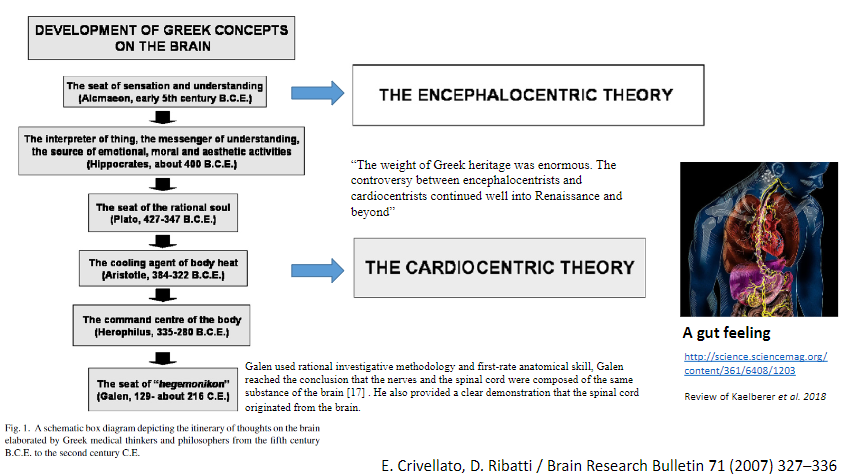
Picture demonstrating the encephalocentric theory and the cardiocentric theory:
-
List the three main neuroimaging techniques and their applications. (3)
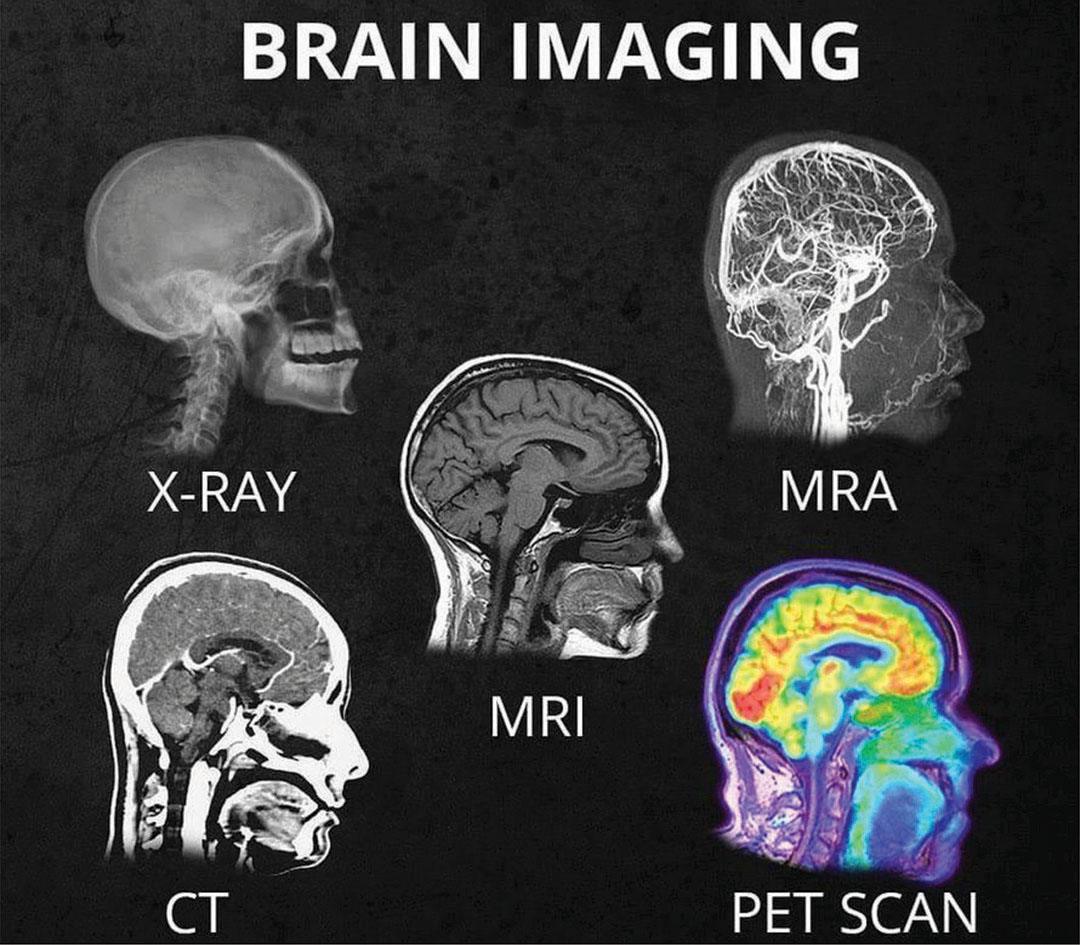
CT: Structural abnormalities like fractures or tumors.
MRI: Detailed soft tissue contrast for conditions like MS.
PET: Functional/metabolic insights, e.g., glucose metabolism in tumors.
-
Describe the cellular structure of cortical grey and white matter. (2)
Grey matter: Contains neuronal cell bodies.
White matter: Composed of myelinated axons.
-
What does the brain's localized functionality and distributed connectivity refer to? (2)
Localized functionality: Specific regions (e.g., sensory/motor homunculus) are dedicated to particular tasks.
Distributed connectivity: Communication between regions through white matter pathways.
-
What signal sources are used in CT, MRI, and PET imaging? (3)
CT: X-rays.
MRI: Magnetic resonance from hydrogen atoms (Radio waves)
PET: Gamma rays from radioactive tracers.
-
How do pathological processes affect neuroimaging results? (2)
Altered tissue structure.
Changes in appearance on images.
-
What systems and diseases are studied in clinical neuroscience? (4)
Systems: Brain, spine, peripheral nervous system, gut.
Diseases: Stroke, tumors, MS, schizophrenia, depression.
-
How can input from the peripheral nervous system (PNS) affect the brain? (1)
Chronic pain alters brain function.
-
List toxins that can affect brain function. (4)
Urea (kidney disease).
Ammonia (liver disease).
Sugar.
Alcohol and nicotine.
-
What behavioral/environmental factors can negatively impact brain function? (3)
Stress.
Isolation.
Excessive internet use.
-
What behavioral factors can boost brain function? (4)
Exercise.
Education.
Enjoyment.
Engagement.
-
What is the significance of Phineas Gage's case in neuroscience? (2)
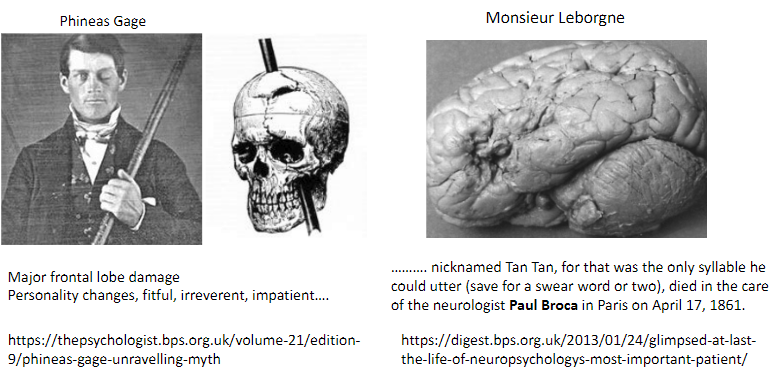
Suffered major frontal lobe damage.
Resulted in personality changes: fitful, irreverent, impatient.
-
Who was Monsieur Leborgne (Tan Tan), and what was his condition? (2)
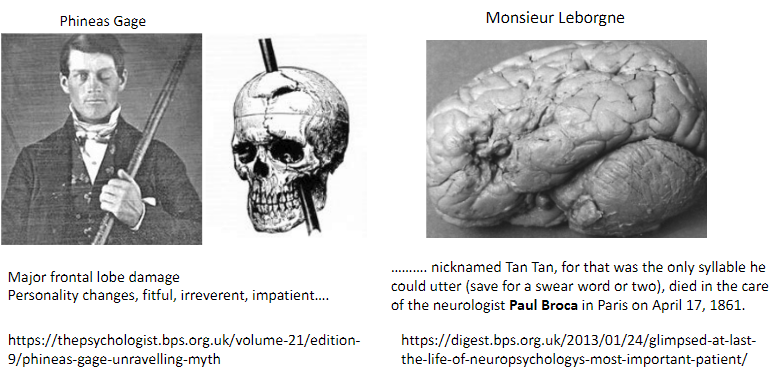
Patient of Paul Broca with severe expressive aphasia.
Could only utter “Tan” and a few swear words.
-
Picture demonstrating pareidolia and how artficial networks are used for image analysis:
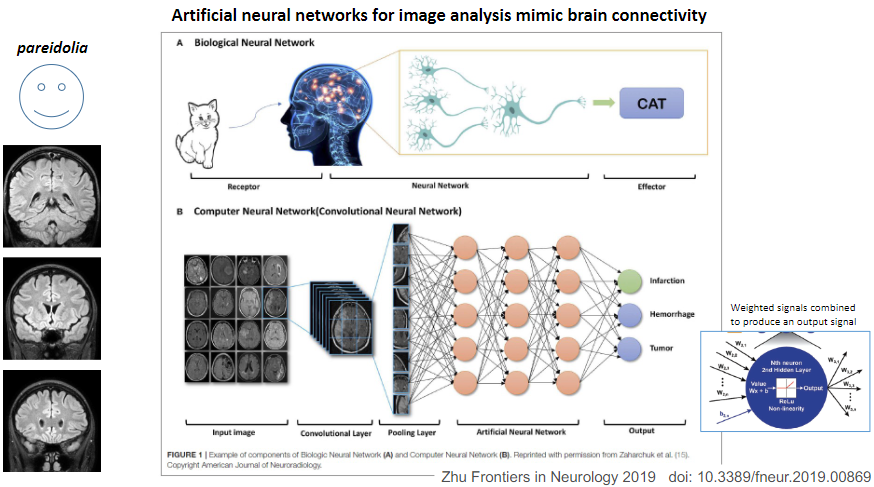
-
What is the function of dendrites in a neuron? (1)
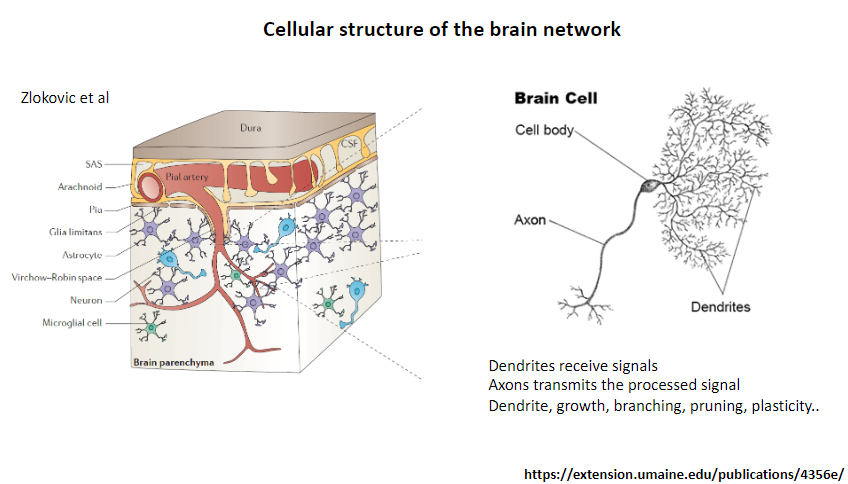
Receive signals from other neurons.
-
What is the function of axons in a neuron? (1)
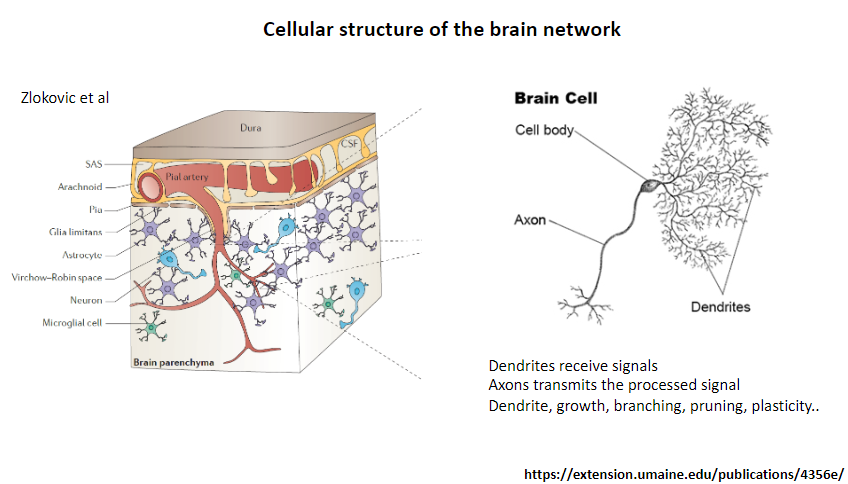
Transmit the processed signal to other neurons or target cells.
-
What processes are associated with dendrites? (4)
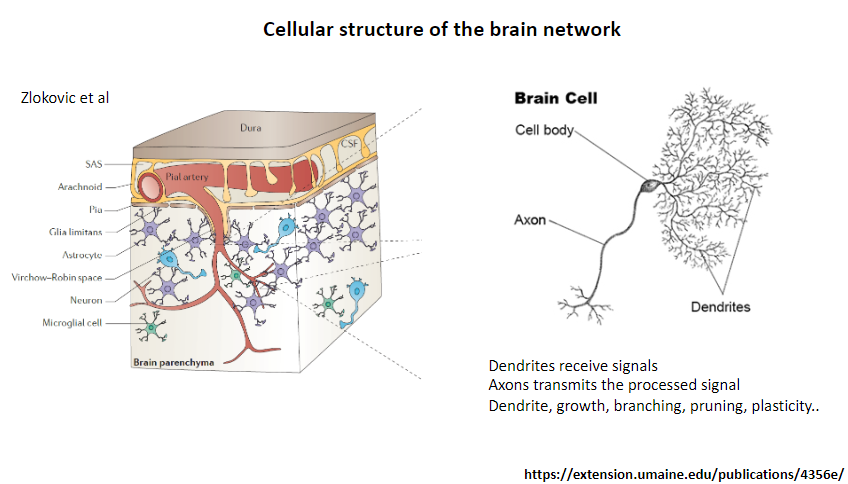
Growth.
Branching.
Pruning.
Plasticity.
-
What is glioblastoma, and what is its key characteristic? (2)
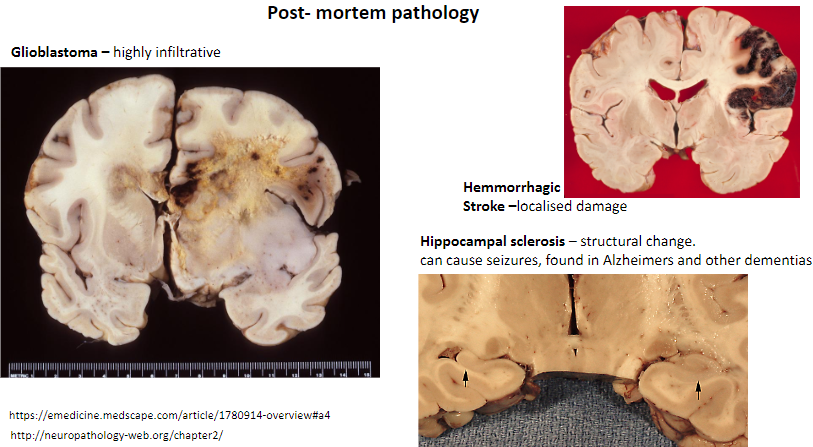
A type of brain tumor.
Highly infiltrative.
-
What is a key feature of a hemorrhagic stroke? (1)
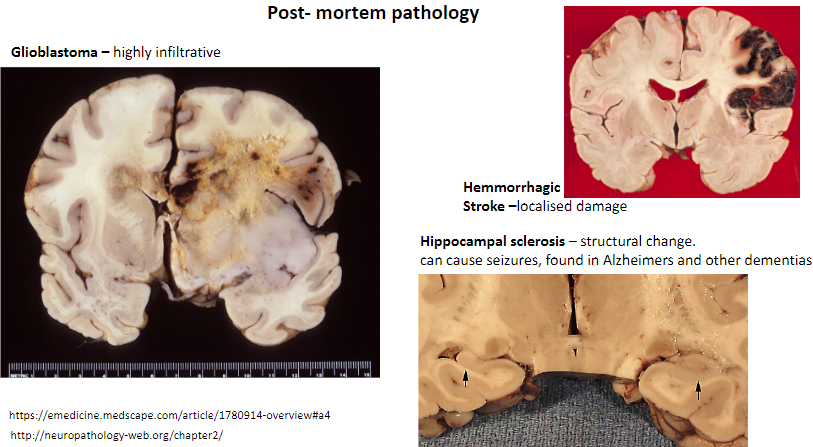
Causes localized damage in the brain.
-
What is hippocampal sclerosis, and what are its effects? (3)
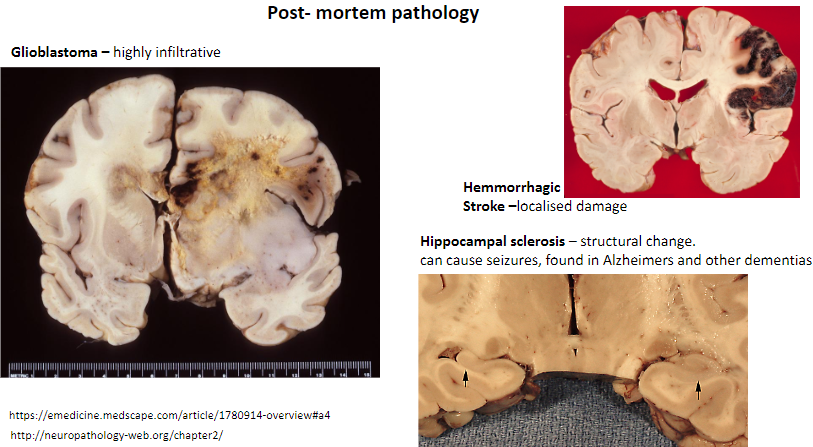
Structural change in the hippocampus.
Can cause seizures.
Found in Alzheimer's and other dementias.
-

Why is the Stroop test challenging, and what does it tell us about processing information? (3)
It requires overriding automatic processes (e.g., reading words) to focus on less automatic tasks (e.g., naming colors).
Demonstrates interference between cognitive processes.
Highlights the brain's reliance on attention and executive control mechanisms.
-
What is an advantage of performing histopathological studies on post-mortem brains? (1)
Provides detailed structural and cellular insights into brain pathology.
-
What is a disadvantage of performing histopathological studies on post-mortem brains? (1)
Limited to changes present at the time of death, which may not fully represent dynamic disease processes.
-
Picture demonstrating medical imaging:
-
Who was Godfrey Hounsfield, and what did he win the Nobel Prize for in 1979? (2)
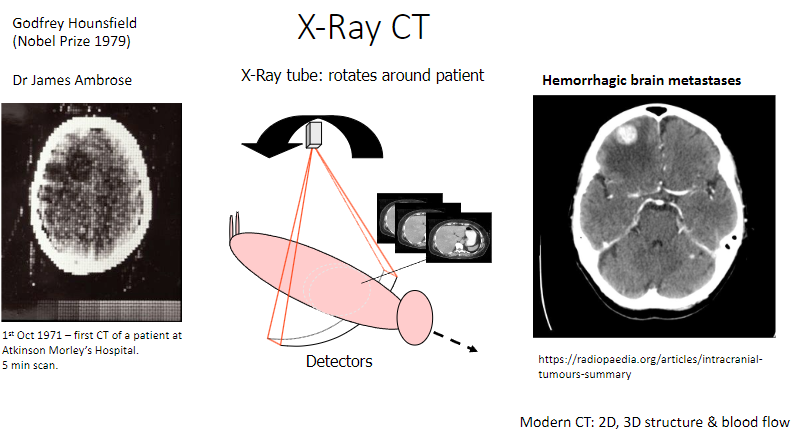
Invented the X-ray computed tomography (CT) scan.
Won the Nobel Prize in Physiology or Medicine in 1979.
-
Who was Dr. James Ambrose, and what was his contribution to CT? (1)
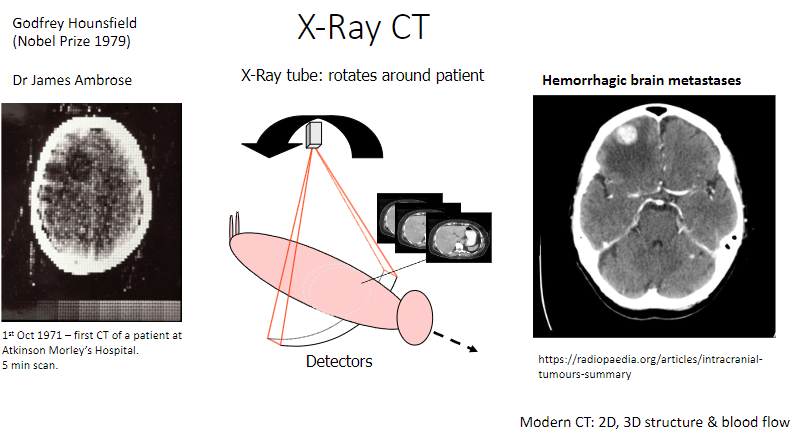
Collaborated with Hounsfield in developing the first CT scanner.
-
How does the X-ray CT scanner work? (2)
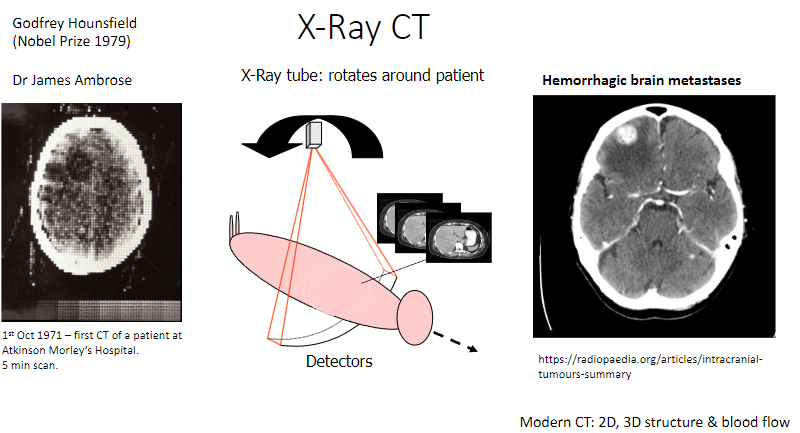
An X-ray tube rotates around the patient to capture images.
Detectors measure the X-rays passing through the body, producing detailed images.
-
What is the significance of October 1st, 1971, in CT history? (1)
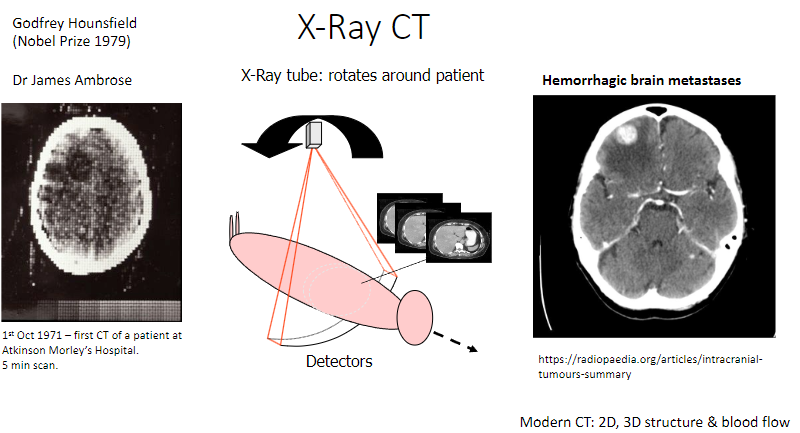
The first CT scan of a patient was performed at Atkinson Morley's Hospital.
-
How long did the first CT scan take? (1)
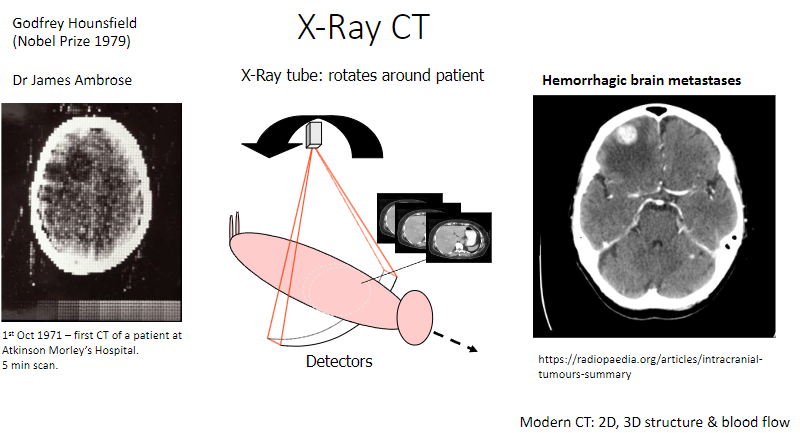
It took 5 minutes to complete the scan.
-
What are modern advancements in CT technology? (3)
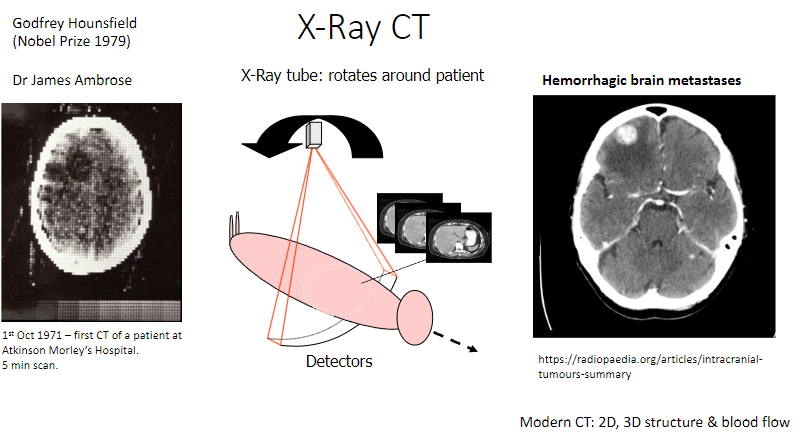
2D imaging: Produces cross-sectional images.
3D imaging: Creates detailed, 3D representations of structures.
Blood flow imaging: Assesses blood circulation in organs like the brain.
-
What role do hydrogen nuclei (H⁺) play in the body? (1)
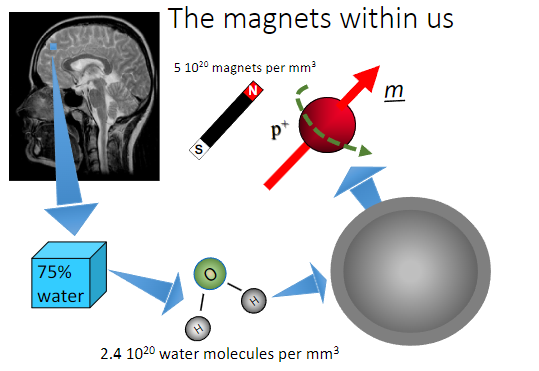
They behave like tiny magnets due to their magnetic properties, which are significant in imaging techniques like MRI.
-
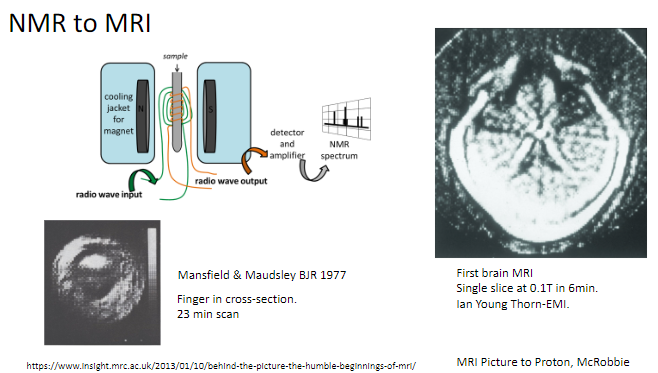
Who were Mansfield and Maudsley, and what did they contribute to MRI? (2)
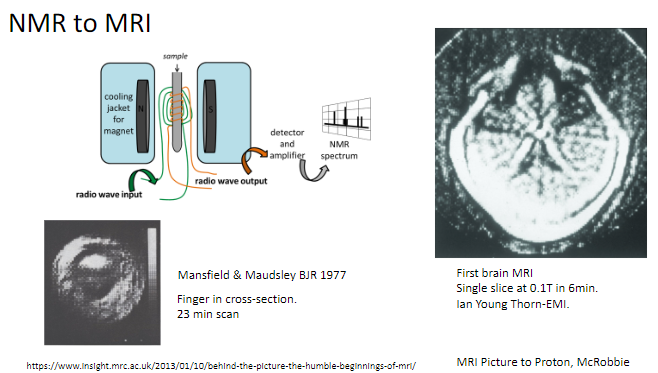
Researchers who published key findings on MRI in the British Journal of Radiology (BJR) in 1977.
Pioneered early imaging techniques using MRI.
-
What was one of the first objects scanned using MRI? (1)
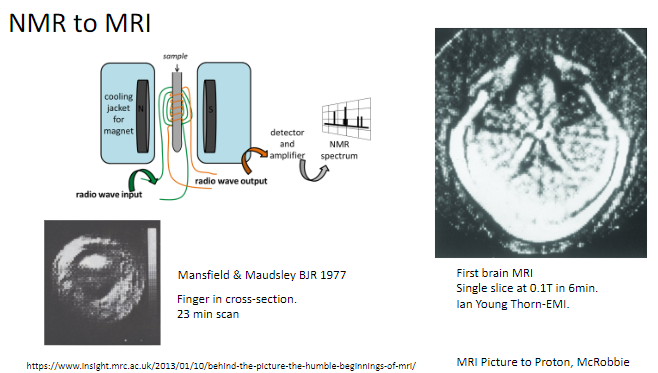
A finger in cross-section, with a scan time of 23 minutes.
-
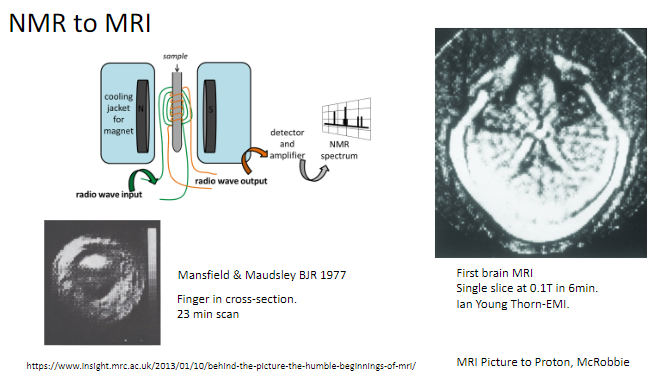
When was the first brain MRI performed, and what were its specifications? (2)
The first brain MRI created a single slice at 0.1 Tesla.
It took 6 minutes to complete.
-
What is the transition from NMR to MRI? (1)
NMR (Nuclear Magnetic Resonance) was adapted for medical imaging, becoming what we now know as MRI (Magnetic Resonance Imaging).
-
What creates magnetisation in tissues during an MRI? (1)
A strong magnetic field.
-
What is the source of magnetisation in tissues for MRI? (2)
Protons in hydrogen found in water and fat within tissues.
-
How is the magnetisation manipulated to produce an MRI signal? (1)
By using radiofrequency (RF) pulses.
-
What are RF pulses? (2)
RF pulses are short bursts of electromagnetic energy in the radiofrequency range that are used in Magnetic Resonance Imaging (MRI) to manipulate the alignment of hydrogen nuclei (protons) in tissues. They play a crucial role in generating the MRI signal and creating an image.
-
What factors influence the intensity of an MRI image? (6)
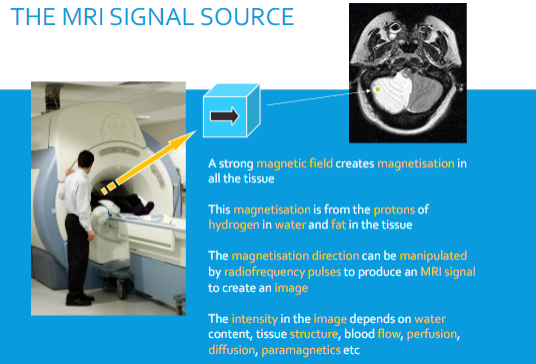
Water content.
Tissue structure.
Blood flow.
Perfusion.
Diffusion.
Presence of paramagnetic substances.
-
What happens to signal intensity on T2-weighted MRI with increased water content? (1)
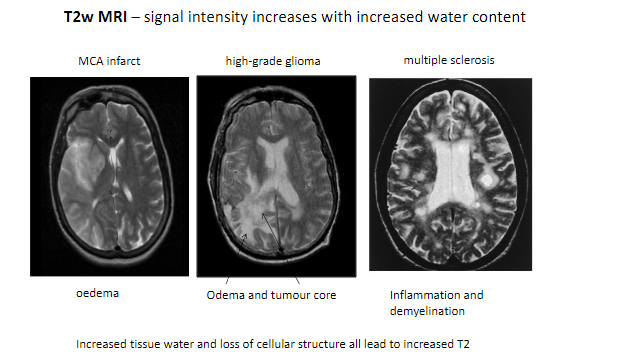
Signal intensity increases with increased water content.
-
What are some conditions detectable with T2-weighted MRI? (5)
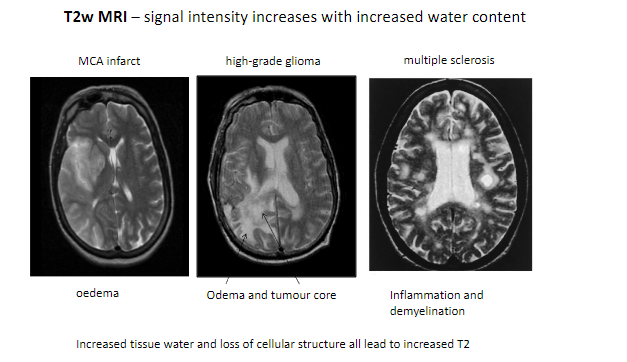
MCA infarct (Middle Cerebral Artery infarction).
High-grade glioma.
Multiple sclerosis.
Edema.
Inflammation and demyelination.
-
What tissue changes contribute to increased T2 signal intensity? (2)
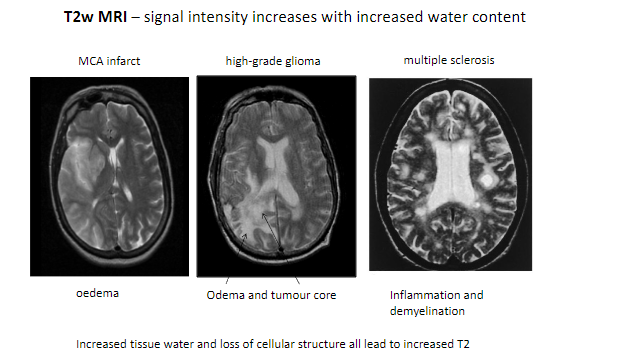
Increased tissue water.
Loss of cellular structure.
-
Which features in pathological tissues result in higher T2 signals? (2)
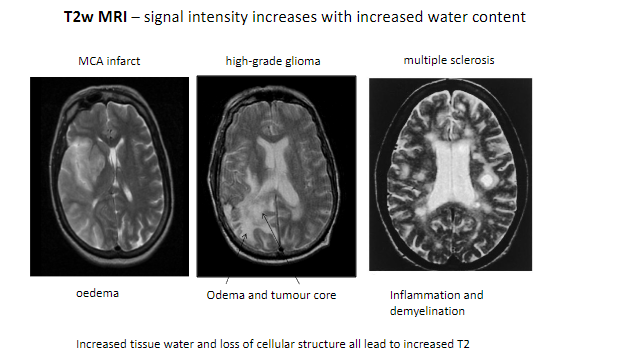
Edema.
Tumor core.
-
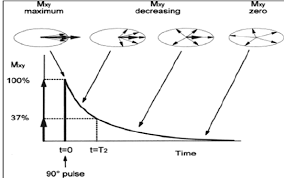
What is T2 in MRI? (1)
T2 refers to the transverse relaxation time, which describes how quickly magnetisation in the transverse plane decays after an RF pulse.
-
What is the primary purpose of MRI in medical imaging? (1)
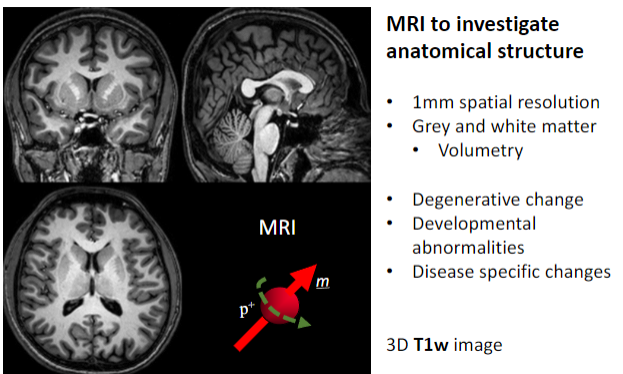
To investigate anatomical structures.
-
What is the spatial resolution of MRI used for anatomical studies? (1)
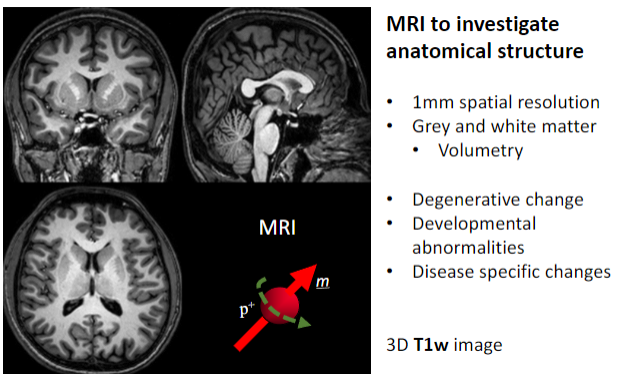
Approximately 1 mm spatial resolution.
-
What brain structures can MRI differentiate? (2)
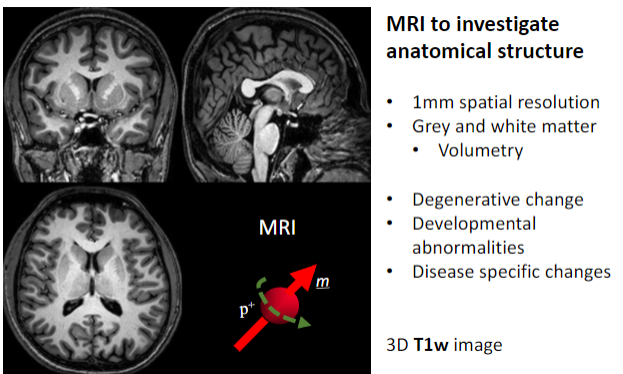
Grey matter.
White matter.
-
What specific applications does MRI have for brain studies? (4)
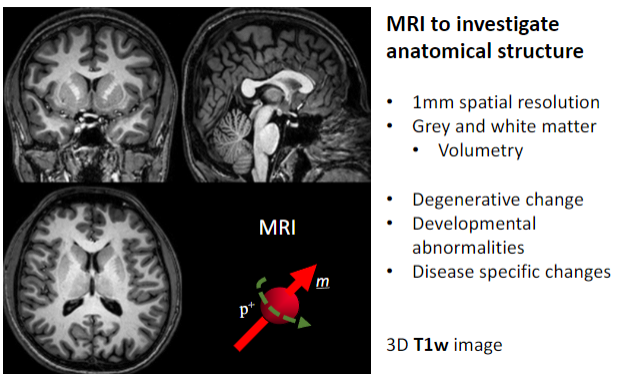
Volumetry (measuring brain volume).
Detecting degenerative changes.
Identifying developmental abnormalities.
Observing disease-specific changes.
-
What imaging sequence is often used for detailed 3D anatomical MRI? (1)
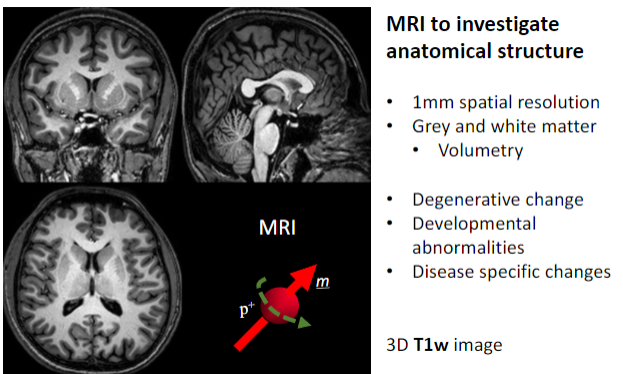
3D T1-weighted (T1w) images.
-
How does water behave in grey matter (GM)? (1)
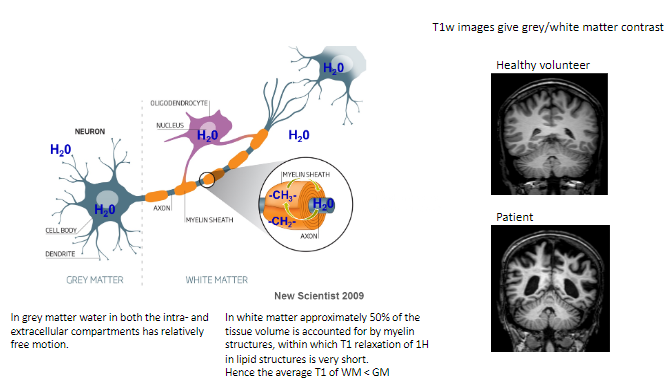
In grey matter, water in both intra- and extracellular compartments has relatively free motion.
-
What proportion of white matter (WM) volume is accounted for by myelin structures? (1)
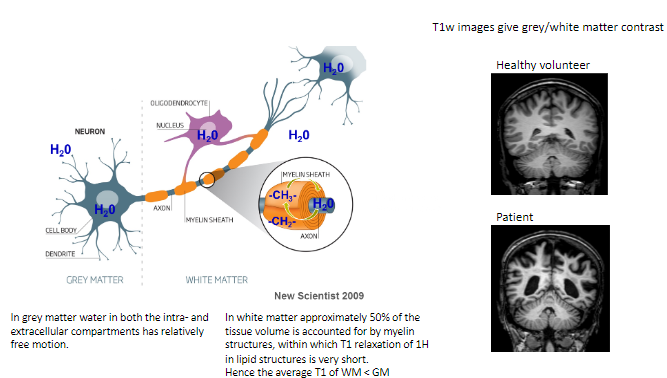
Approximately 50% of the tissue volume in white matter consists of myelin structures.
-
What is unique about T1 relaxation in myelin's lipid structures? (1)
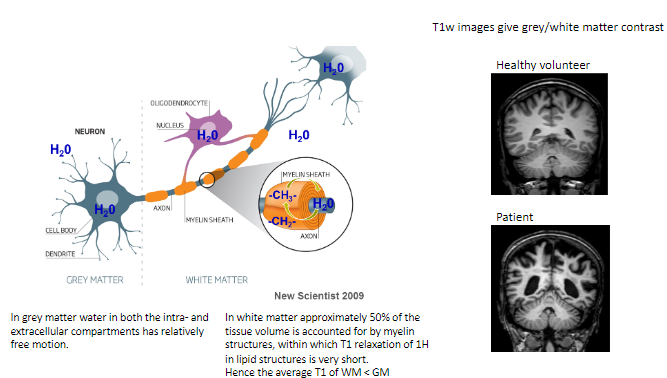
The T1 relaxation of hydrogen (^1H) in the lipid structures of myelin is very short.
-
How does the average T1 of white matter compare to grey matter? (1)
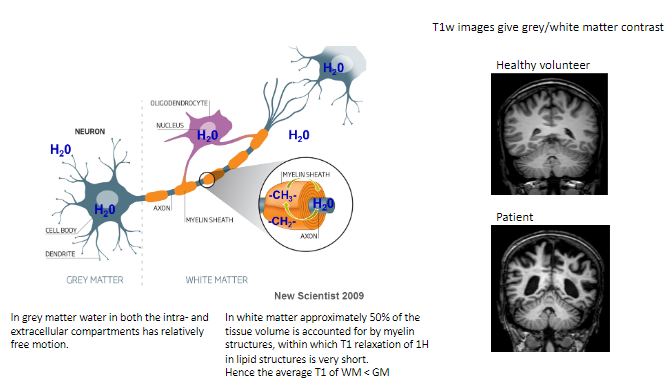
The average T1 of white matter is shorter than grey matter.
-
What type of contrast do T1-weighted images provide? (1)
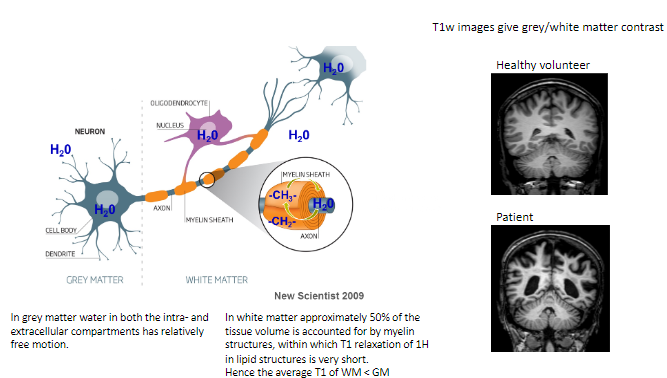
T1-weighted images give grey/white matter contrast, helping differentiate between these tissues.
-
How does T1-weighted imaging differ between healthy volunteers and patients? (1)
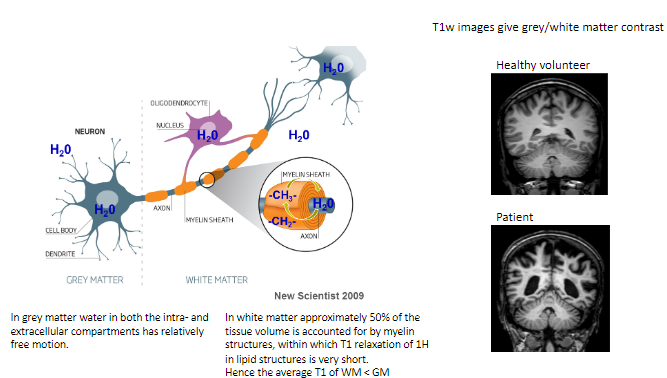
Healthy volunteers show normal grey/white matter contrast, whereas patients may exhibit abnormalities indicating disease or damage.
-
What is synaptic pruning? (1)
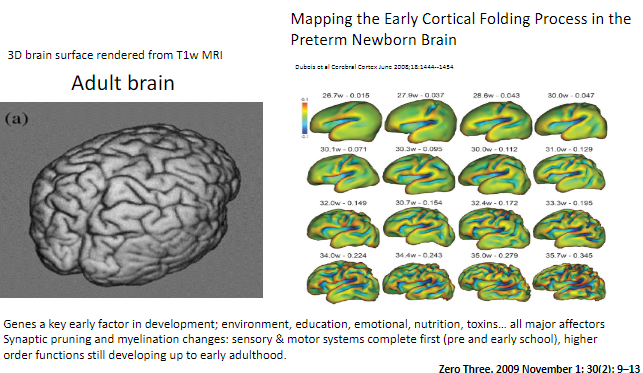
Synaptic pruning is the process where excess synapses (connections between neurons) are eliminated to improve the efficiency of neural networks.
-
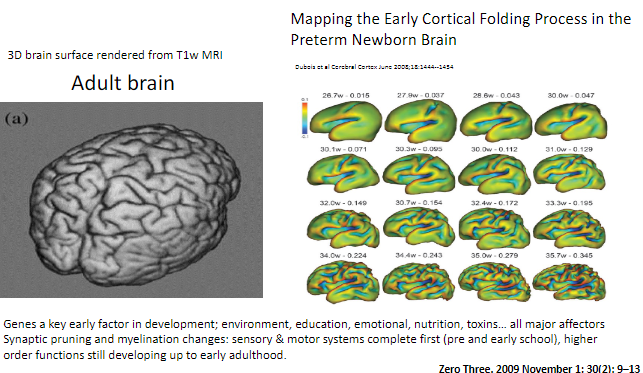
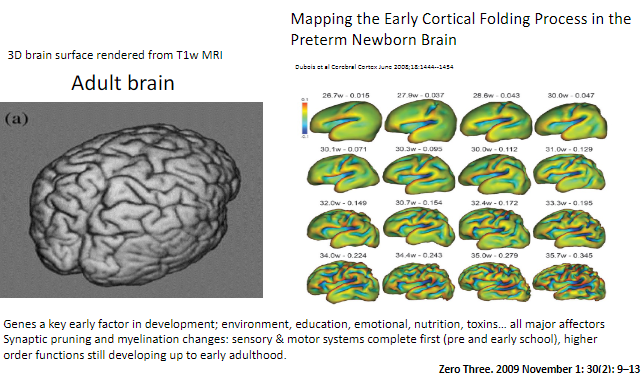
When do sensory and motor systems complete synaptic pruning and myelination? (1)
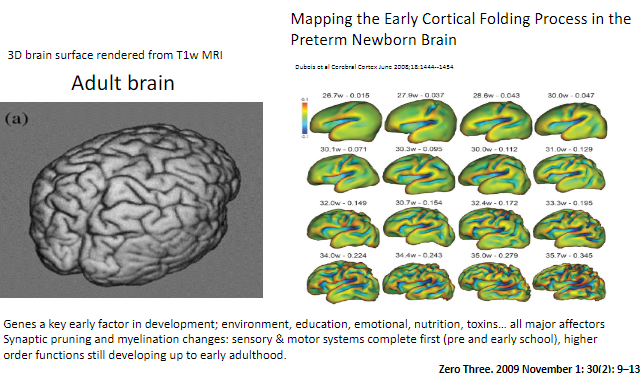
Sensory and motor systems are complete by pre- and early school years.
-
When do higher-order functions continue developing? (1)
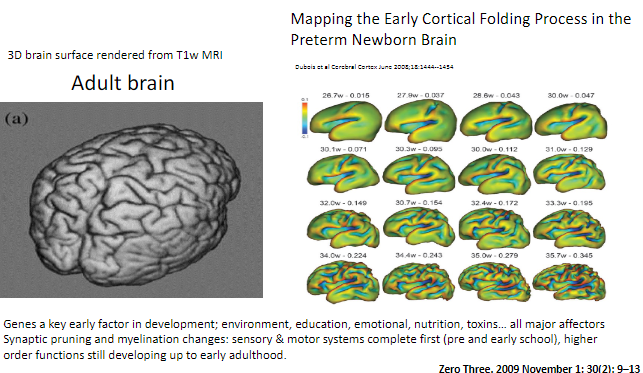
Higher-order functions, such as those involving complex cognitive abilities, continue developing up to early adulthood.
-
What factors affect brain development? (5)
Genes: Key early factors in development.
Environment: Surrounding conditions influencing growth.
Education: Learning experiences and cognitive development.
Emotional health: Emotional experiences impacting brain function.
Nutrition & toxins: Diet and exposure to harmful substances influencing brain development.
-
What systems are involved in early brain development, and how are they affected? (2)
Sensory and motor systems develop earlier, while higher-order functions continue developing later into early adulthood.
-
How can synaptic pruning and myelination be visualized in the brain? (1)
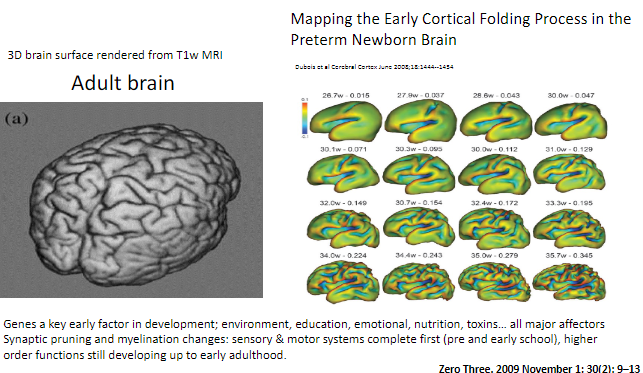
Brain development changes such as synaptic pruning and myelination can be observed using T1-weighted MRI, with 3D renderings showing cortical folding processes.
-
What does high anisotropy in water diffusion indicate in white matter? (1)
High anisotropy in water diffusion indicates highly directional movement of water molecules, which is seen in healthy white matter tracts.
-
What is measured using Diffusion MRI in white matter? (2)
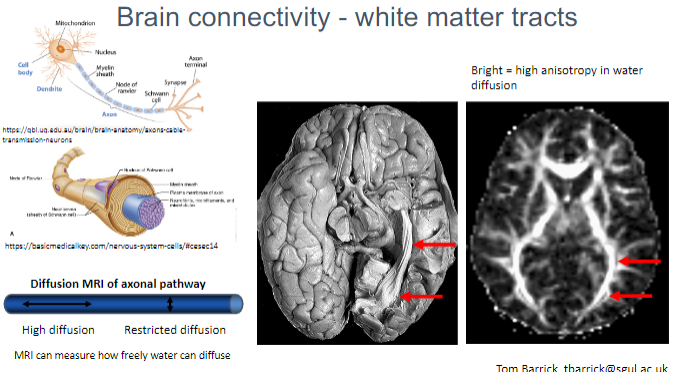
Water diffusion within the axonal pathways of white matter.
The degree of restricted or free diffusion of water, which helps assess brain connectivity.
-
What is the significance of restricted vs. high diffusion in Diffusion MRI? (2)
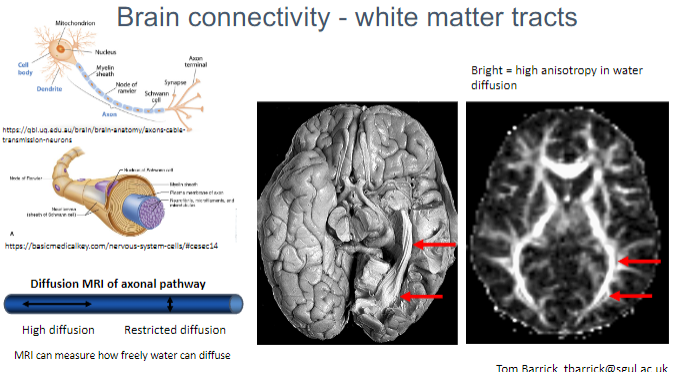
High diffusion indicates more freedom for water molecules to move, typically seen in healthy white matter.
Restricted diffusion suggests damage or disruption of axonal pathways.
-
What do white matter tracts represent in brain connectivity? (1)
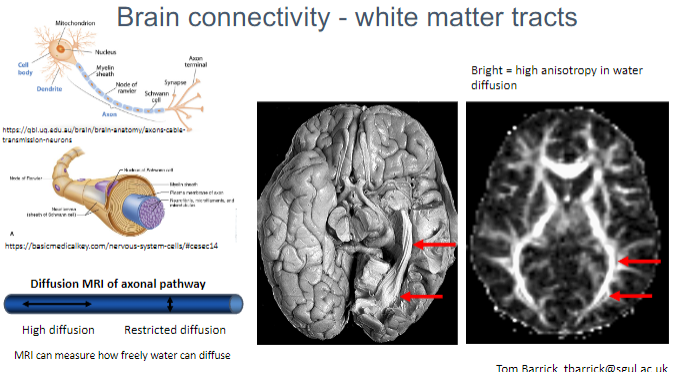
White matter tracts represent the connections between different regions of the brain, facilitating communication between neurons.
-
What does MRI measure in relation to water diffusion in axonal pathways? (1)
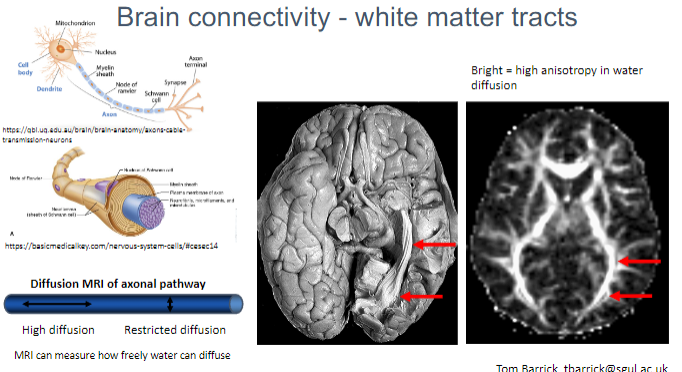
MRI can measure how freely water molecules diffuse along the direction of the axonal pathways, helping to map brain connectivity.
-
What is the purpose of Diffusion MRI in brain studies? (1)
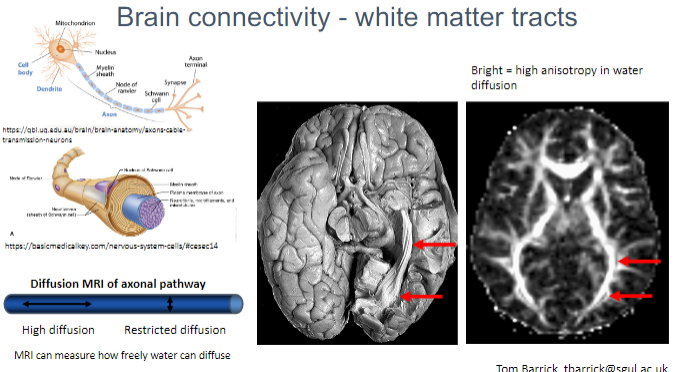
Diffusion MRI helps to visualize and quantify the orientation and integrity of white matter tracts, offering insights into brain connectivity.
-
Picture demonstrating the anatomy of white matter:
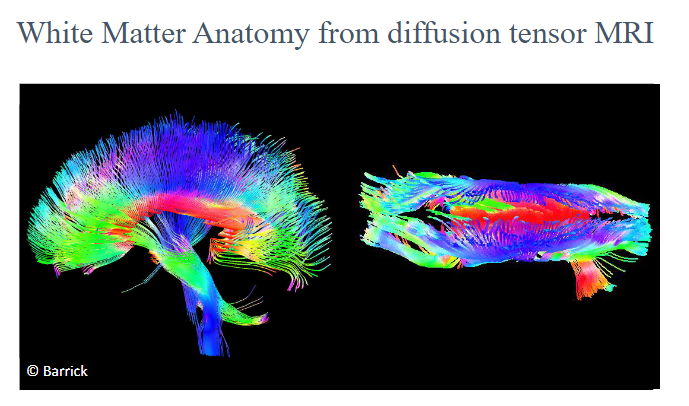
-
Picture demonstrating the Displacement of the corticospinal tract by a brain tumour:
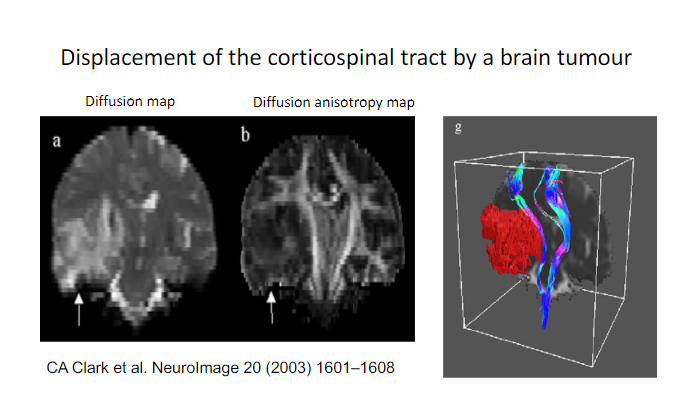
-
What pathological/structural changes in brain tissue cause lesions to appear bright on T2-weighted images? (3)
Increased water content: Conditions like edema, inflammation, or tumours increase the water content of tissues, making them appear bright on T2 images.
Loss of cellular structure: Diseases that lead to structural changes (e.g., demyelination, necrosis) result in altered tissue composition, enhancing T2 signal.
Increased tissue water: Pathological processes, such as swelling, lead to a higher water content, which gives a higher signal intensity on T2-weighted images.
-
Describe two physical/structural properties of neurones that enable uniquely different types of MR images to be acquired. (2)
Water content: The varying amount of water in grey and white matter affects signal intensity on MR images (e.g., T1 and T2 contrasts).
Myelination: The presence of myelin in white matter impacts MRI signals due to the different relaxation times of hydrogen in lipid-rich structures, which results in distinct contrasts in MR images.

