-
what is a standard solution
a standard solution is one of known concentration
-
how is molar concentration determined
The molar concentration of a solution is determined by the amount of solute and the volume of solution.
-
what is molar volume
The molar volume of an ideal gas is a constant at specified temperature and pressure
-
what does avogadros law enable
Avogadro's law enables the mole ratio of reacting gases to be determined from
volumes of the gases
-
how are masses of atoms expressed
Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/molecular mass (Mr)
-
how is molar mass defined
Molar mass can be defined as the mass in 1 mol → unit is g/mol
-
Explain the convergence of lines in a hydrogen emission spectrum.
energy levels are closer together at high energy / high frequency / short wavelength
-
State what can be determined from the frequency of the convergence limit.
ionisation energy
-
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
Anode (positive electrode):
2Cl–→ Cl2 (g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e–→ Mg (l)
-
Magnesium chloride can be electrolysed.
Identify the type of reaction occurring at the cathode (negative electrode).
reduction
-
Magnesium chloride can be electrolysed.
State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
Anode (positive electrode):oxygen/O2
Cathode (negative electrode):hydrogen/H2
-
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
inert Pt electrodeplatinum black conductor
1 mol dm–3 H+(aq)
H2 (g) at 100 kPa
-
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
Mg(s) + Cu2+ (aq) → Mg2+ (aq) + Cu(s)
-
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq)
Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
+0.34V –(–2.37V) = +2.71 V
-
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
cell potential increases
potential of the copper half-cell increases/becomes more positive
-
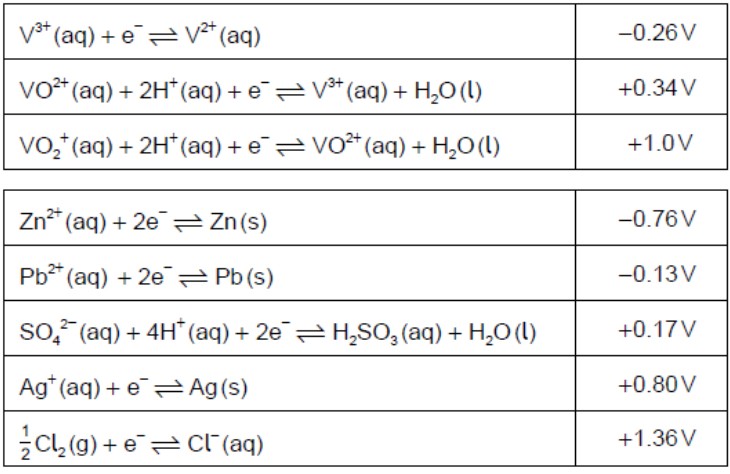
Determine the oxidation state of vanadium in each of the following species.
V2O5:+5VO2+:+4
-
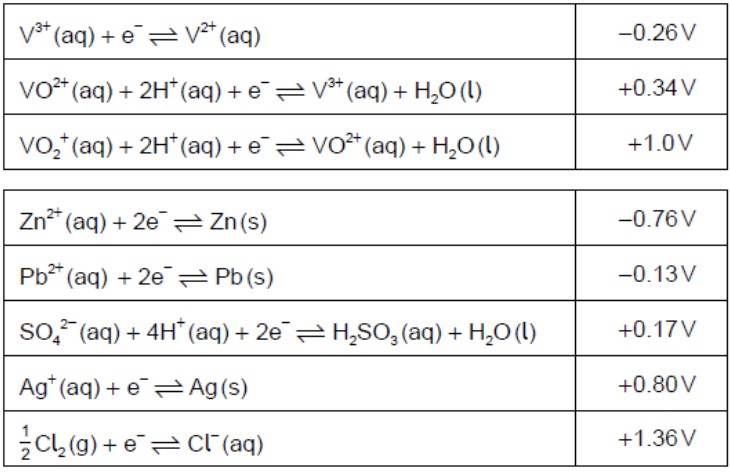
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) toV3+(aq) but no further.
H2SO3(aq)ORPb(s)
-
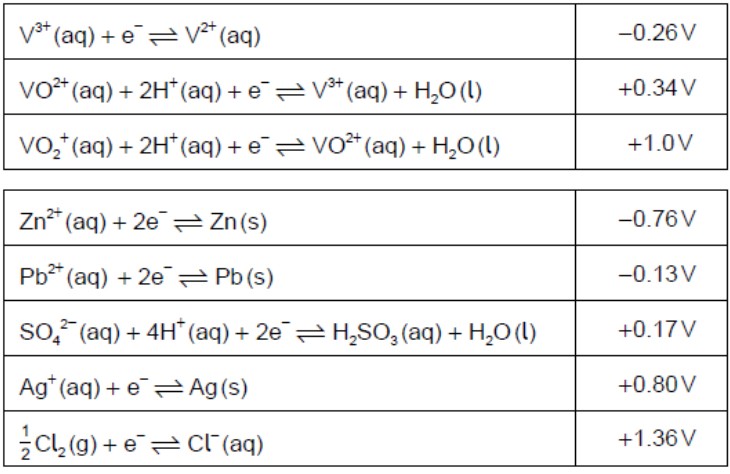
Identify, from the table, a non-vanadium species that could convert VO2+(aq)toV2+(aq).
Zn(s)
-
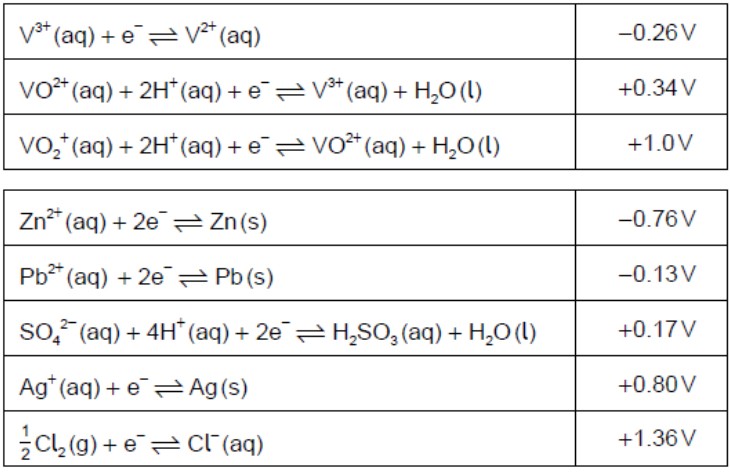
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solutionto form V3+(aq).
VO2+(aq)+V2+(aq)+2H+(aq)→2V3+(aq)+H2O
-
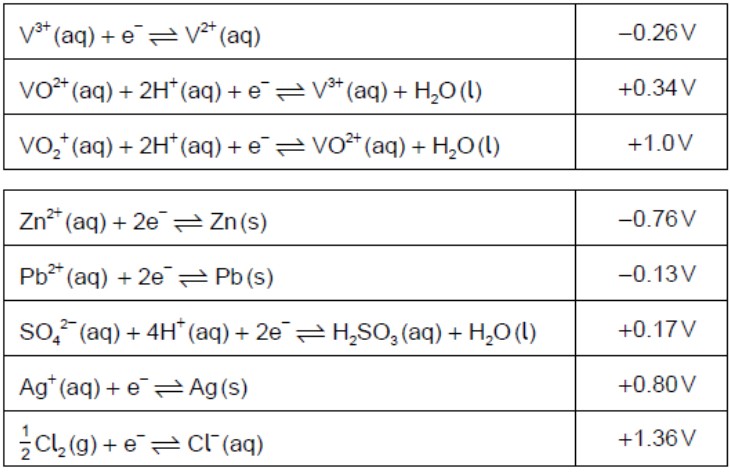
Comment on the spontaneity of this reaction by calculating a value for ΔGθusingthe data given in (b) and in section 1 of the data booklet.
Eθ = +0.34V−(−0.26V) =+0.60V
ΔGθ=−nFEθ=−9.65×104C mol−1×0.60JC−1=
spontaneous as ΔGθis negative

