-
nociceptorsnerve endings designed to detect painful sensations form the peripher and transmit them to the CNS. Located within the skin, joints, connective tissue, muscle, and thoracic, abdominal, and pelvic viscera.
-
A (beta) fibersA fibers are myelinated and large in diamet, they transmit the pain signal rapidly to the CNS.
-
C fibersUnmyelinated and smaller, transmit signals more slowy. Secondary sensations are diffuse and aching, last longer after initial injury.
-
Interneuronsa neuron that transfers pulses between neurons
-
Substantia gelatinosalamina II. Receives sensory input from various ares of the body.
-
Anterolateral spinothalamic tractWhen pain signals cross to the spinal cord they ascend the brain by this tract.
-
Nociceptive painwhen functioning and intact nerve fibers in the periphery and the CNS are stimulated. Triggered outside the nervous system from actual or potential tissue damage. Four phases: Transduction transmission perception modulation
-
transductionnoxious stimulus in the form of traumatic or chemical injury, burn, incision, or tumor takes place in the periphery. Release chemical including substance P, histamine, Prostaglandins, serotonin, and bradykinin. Transmit pain message or action potential along sensory afferent nerve fibers to the spinal cord. terminate in dorsal horn of spinal cord and carried by a second set inclduing substance P, glutamate, and adenosine triphosphate.
-
TransmissionPain impulse moves from the level of the spinal cord to the brain. This is where opiiod receptors can block pain signaling if administered to paitent. Pain impulse moves to brain via ascending fibers. After hitting thalamus the message is dispersed to higher cortical areas via mechanism.
-
Perceptionsignifies the conscious awareness of a painful sensation. Cortical structures such as the limbic system create emotional response.
-
Modulationbuilt in mechaism that slows down and stops the processing of painful stimulu.
-
Neuropathic painpain that does not adhere to the typical and rather predictable phases in nociceptive pain. Pain due to lesion or disease. Abnormal processing of the pain message. Most difficult to assess and treat. Evolves into chronic pain. Nerve cells are altered making them more sensitive to any future stimulus. Examples include, Diabetes mellitus, herpes zoster, HIV, sciatic, trigeminal neuralgia, phatom limb pain, and chemotherapy, lesions, stroke multiple sclerosis, and tumor. Can be identified somewhat by functional MRI (fMRI).
-
Visceral painoriginates from the larger organs. Dull, squeezing, deep, or cramping. Either direct injury or tumor, ischemia, distention or contraction.
-
Somatic painoriginates from the muscloskeletal tissues or the body surface.
-
Deep somatic painpain from blood vessels, joints, tendons, muscles, and bones. May result from pressure, trauma, or ischemia.
-
Cutaneous painderived from skin surface and subcutaneous tissues. superficial, sharp, or burning.
-
Referred painpain felt at a site but it actually originates from a different area. Difficult for the brain to differentiate the point of origin. May originate from visceral or somatic structures.
-
Acute painshort term self-limit pain from a predicatable trajectory. Dissipates after the injury heals.
-
Chronic paindiagnosed when pain continues for 6 months or longer. Malignant (cancer-related) nonmalignant. Doesn't stop when injury heals. persists after predicted trajectory. Originates from abnormal processing of pain fibers from peripheral or central sites.
-
Breakthrough paintransient spike in pain level, moderate to severe in intensity, in an otherwise controlled pain syndrome.
-
Incident pain
is an acute type that happens predictably when certain movements take place.
-
malignant pain vs nonmalignant pain
chronic pain that is derived from cancer or not related to cancer
-
Pain and the aging adult
Older adults may have additional fears about becoming dependent, undergoing invasive procedures, taking pain medications, and having a financial burden. The most common pain-producing conditions for aging adults include pathologies such as osteoarthritis, osteoporosis, peripheral vascular disease, cancer, peripheral neuropathies, angina, and chronic constipation.
Dementia does not impact the ability to feel pain, but it does impact the person's ability to effectively use self-report instruments.
-
gender differences pain
Gender differences are influenced by societal expectations, hormones, and genetic makeup
-
OPQRST
onset, provocation/palliation, quality/quantity, region/radiation, severity, timing
-
Initial pain assesment
Clinician asks patients eight questions concerning location, duration, quality, intensity, and aggravating/relieving factors. Furthermore, clinician adds questions about manner of expressing pain and effects of pain that impairs one’s quality of life.
-
Brief pain inventor
Clinician asks patient to rate pain within past 24 hours on graduated scales (0 to 10) with respect to its impact on areas such as mood, walking ability, and sleep.
-
Short-form McGill Pain Questionnaire
Clinician asks patient to rank list of descriptors in terms of their intensity and to give an overall intensity rating to his or her pain.
-
Pain rating scales
one-dimensional and are intended to reflect pain intensity. can indicate a baseline intensity, track changes, and give some degree of evaluation to a treatment modality. There are different subtypes that use numbers, verbal description, visual analog, or descriptor scale.
Selection is based on patient understanding and age of development.
-
Numeric rating scales
patient to choose a number that rates level of pain, with 0 being no pain and highest anchor 10 indicating worst pain.
-
verbal descriptor scales
have the patient use words to describe pain
-
visual analog scales
have the patient mark the intensity of the pain on a horizontal line from “no pain” to “worst pain.
-
descriptor scales
in which patients are asked to indicate their pain by using selected pain term words
-
Pain children and toddlers
preverbal and incapable of self-report, pain assessment is dependent on behavioral and physiologic cues.
It is important to underscore understanding that infants do feel pain.
Children 2 years of age can report pain and point to its location but cannot rate pain intensity.
It is helpful to ask parent or caregiver what words the child uses to report pain.
-
joints
Note size, contour, and circumference of joint.
Check active or passive range of motion.
Joint motion normally causes no tenderness, pain, or crepitation.
-
muscle and skin
Inspect skin and tissues for color, swelling, and any masses or deformity.
-
abdomen
Observe for contour and symmetry.
Palpate for muscle guarding and organ size.
Note any areas of referred pain.
-
acute pain behaviors
Involve autonomic responses
Protective purpose
Individuals experiencing moderate to intense levels of pain may exhibit the following behaviors:
Guarding, grimacing
Vocalizations such as moaning, agitation, restlessness, stillness
Diaphoresis,
Change in vital signs
-
chronic pain behaviors
Persistent (Chronic) pain behaviors
Often live with experience for months and years
Adaptation occurs over time.
Clinicians cannot look for or anticipate the same acute pain behaviors to exist in order to confirm a pain diagnosis.
Shows more variability than acute pain behaviors
Higher risk for under detection
Associated behaviors:
Bracing, rubbing
Diminished activity
Sighing
Change in appetite
-
Cries Score
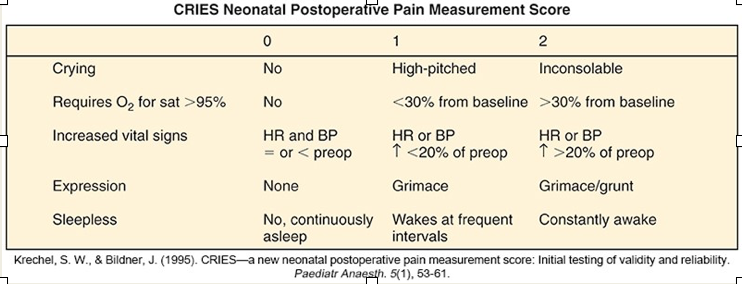
Measures postoperative pain in preterm and term neonates
Examines physiologic and behavioral indicators on 3 point scale
-
FLACC
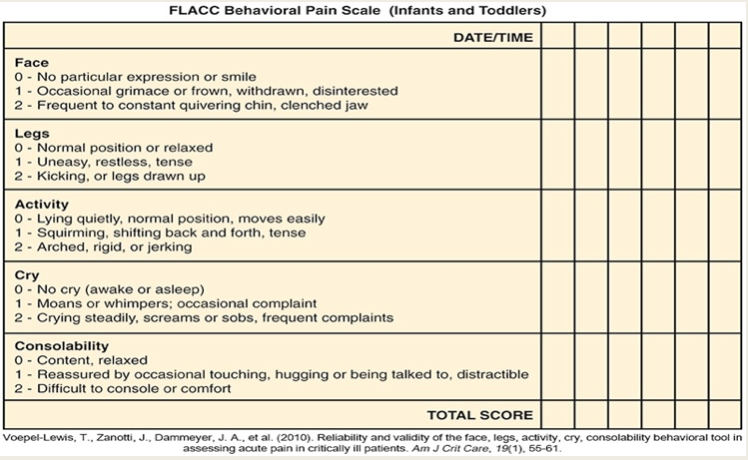
Nonverbal tool used for infants and young children up to age 3
Assesses 5 behaviors of pain (facial expression, leg movement, activity level, cry, and consolability)
-
PAINAD
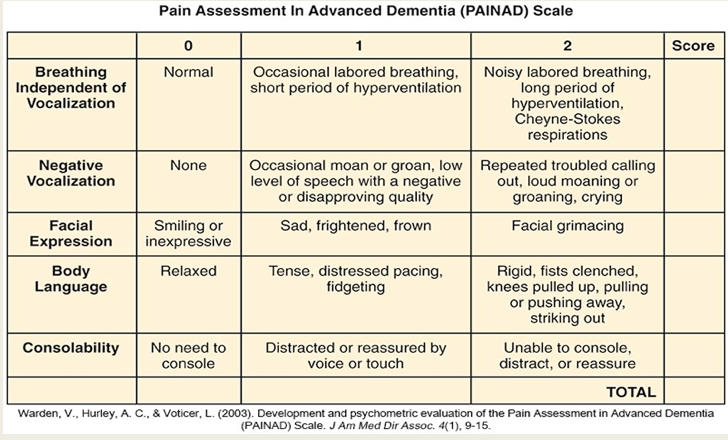
Evaluates 5 common behaviors
Breathing, vocalization, facial expression, body language, and consolability
Quantified behaviors in category 0 to 2
Total score metric 0 to 10
Score of 4 or more requires treatment.

