Haemoglobin structure and function (Pathology)
Further Reading: Hoffbrand's Essential Haematology. Hoffbrand and Moss, Wiley-Blackwell, 7th Ed. 2016
-
State some information about Haemoglobin (4)
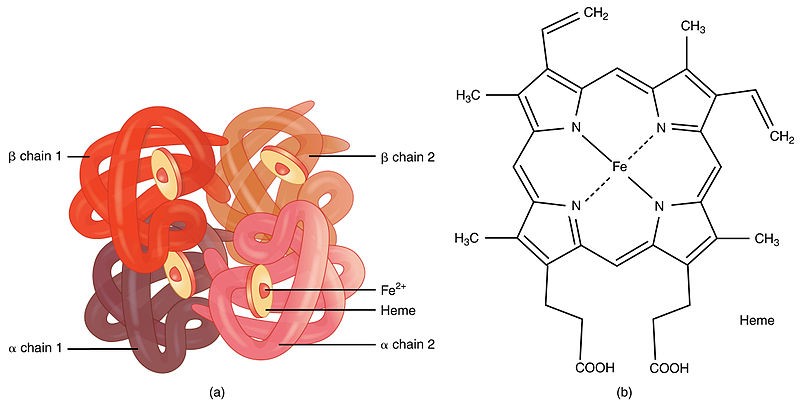
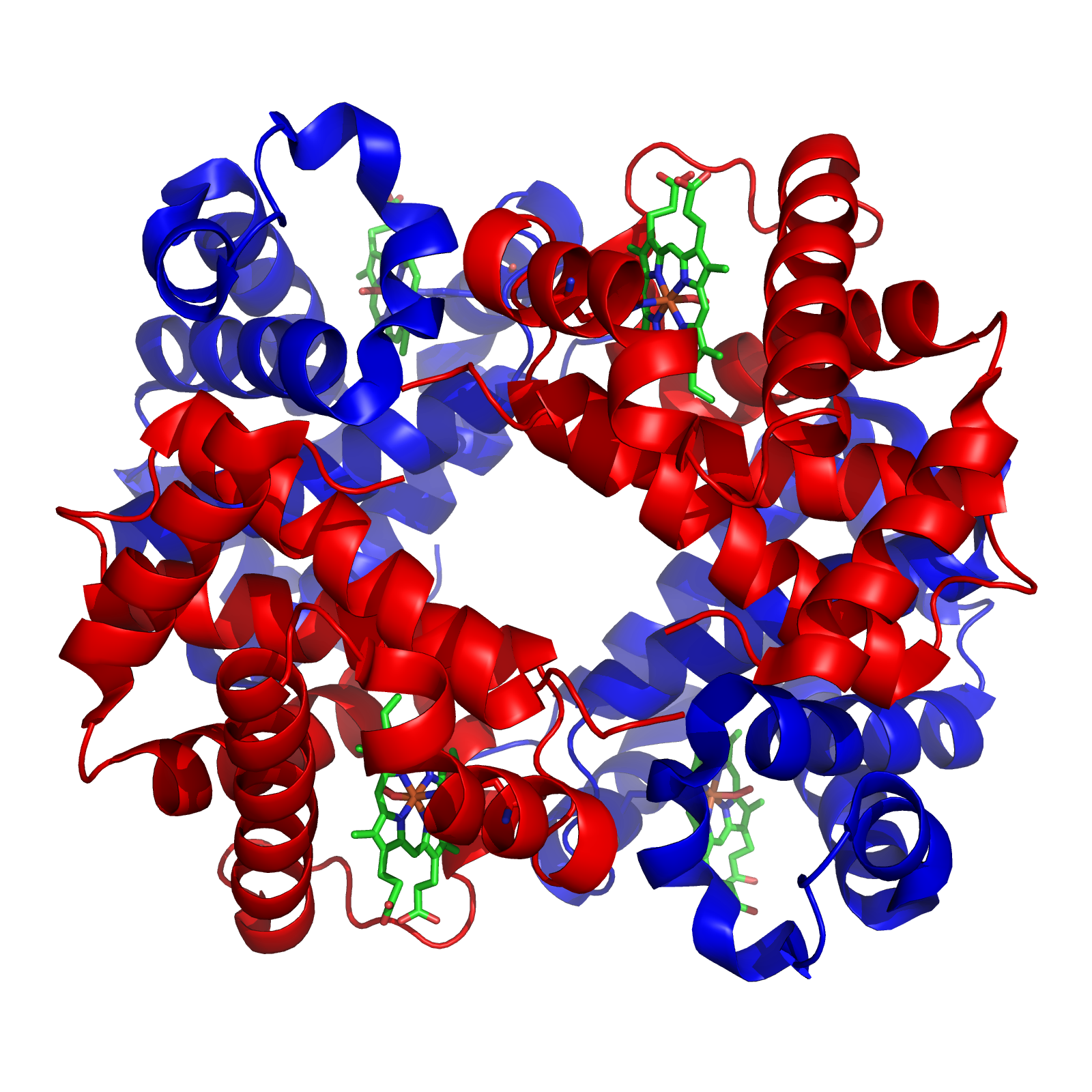
-Haemoglobin (Hb) is a globular haemoprotein made up of 1/3 of red blood cells
-Haemoproteins are a group of specialized proteins that contain haem as a tightly bound prosthetic group
-Haem is a complex of protoporphyrin IX and ferrous iron (Fe2+)
-Iron held in the centre of haem molecule by bonds to the 4 nitrogen of a porphyrin ring
+The iron has 6 co-ordinations; 4 binds pyrrol rings, one binds with histidine and one is free to bind O2
-
How much and where is Hb synthesised (%)?
-65% of the Hb is synthesized in the erythroblasts, and 35% at the reticulocyte stage
-
What is the normal concentration of Hb in the blood (both Females and Males)?
Normal conc. of Hb in blood:Adult female 11.5 – 15.5 g/dlAdult male: 13.5 – 17.5 g/dl
-
What is Hb production regulation stimulated by?
Stimulated by tissue hypoxia
-
What does hypoxia do?
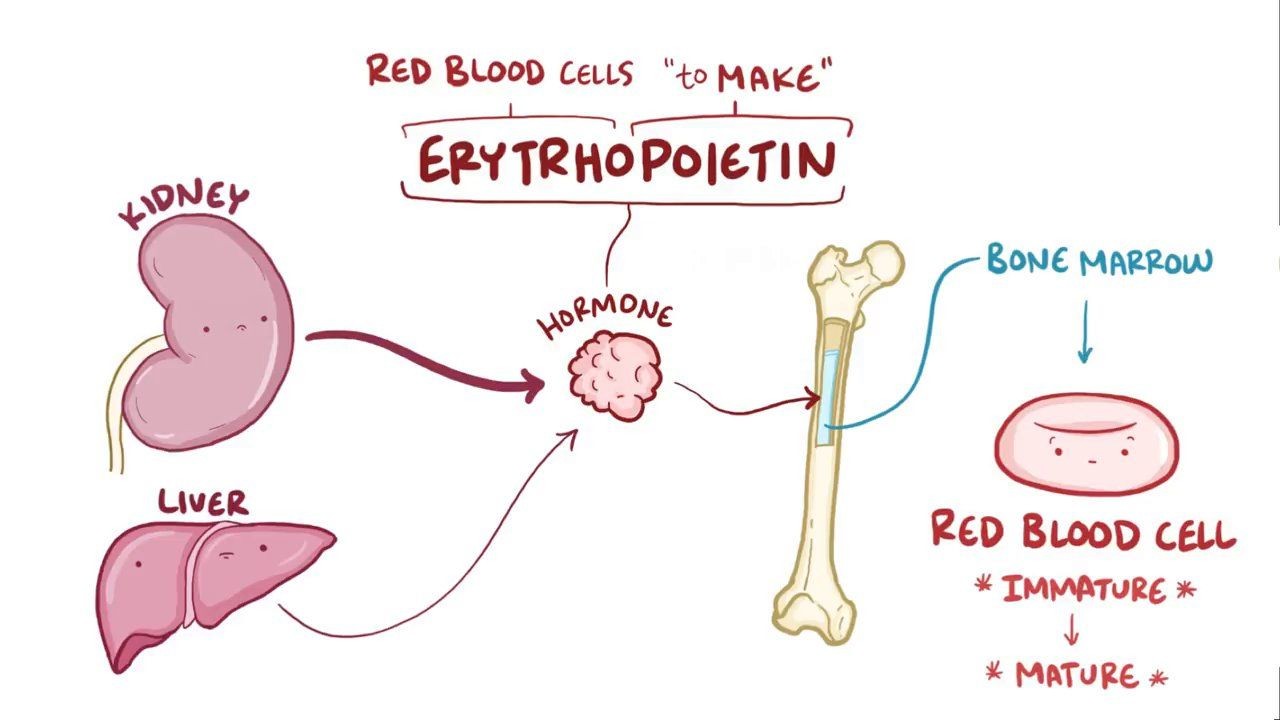
Hypoxia causes the kidneys to increase production of EPO (Erythropoietin)which increases RBC and Hb production
-
Haem synthesis occurs largely where?
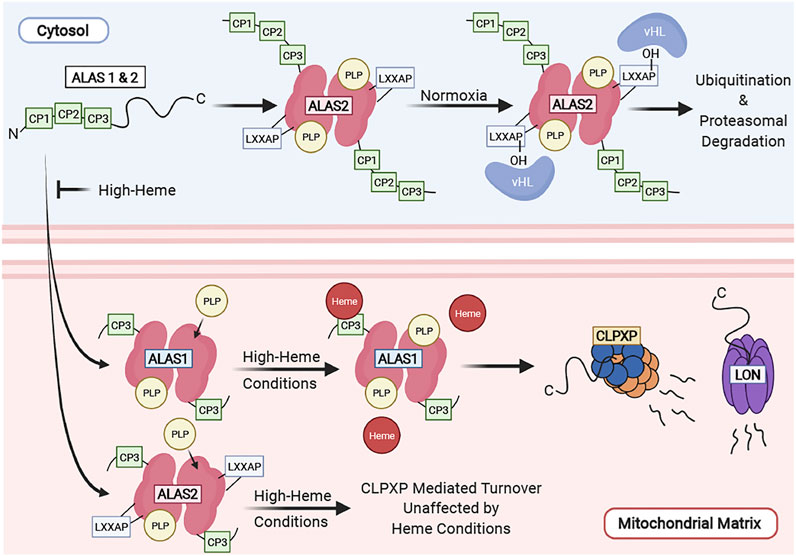
Occurs largely in the mitochondria, by chain of events
-
When it comes to iron delivery and supply, where is iron delivered to the red cell by?
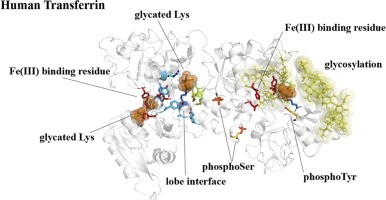
Via transferrin
-
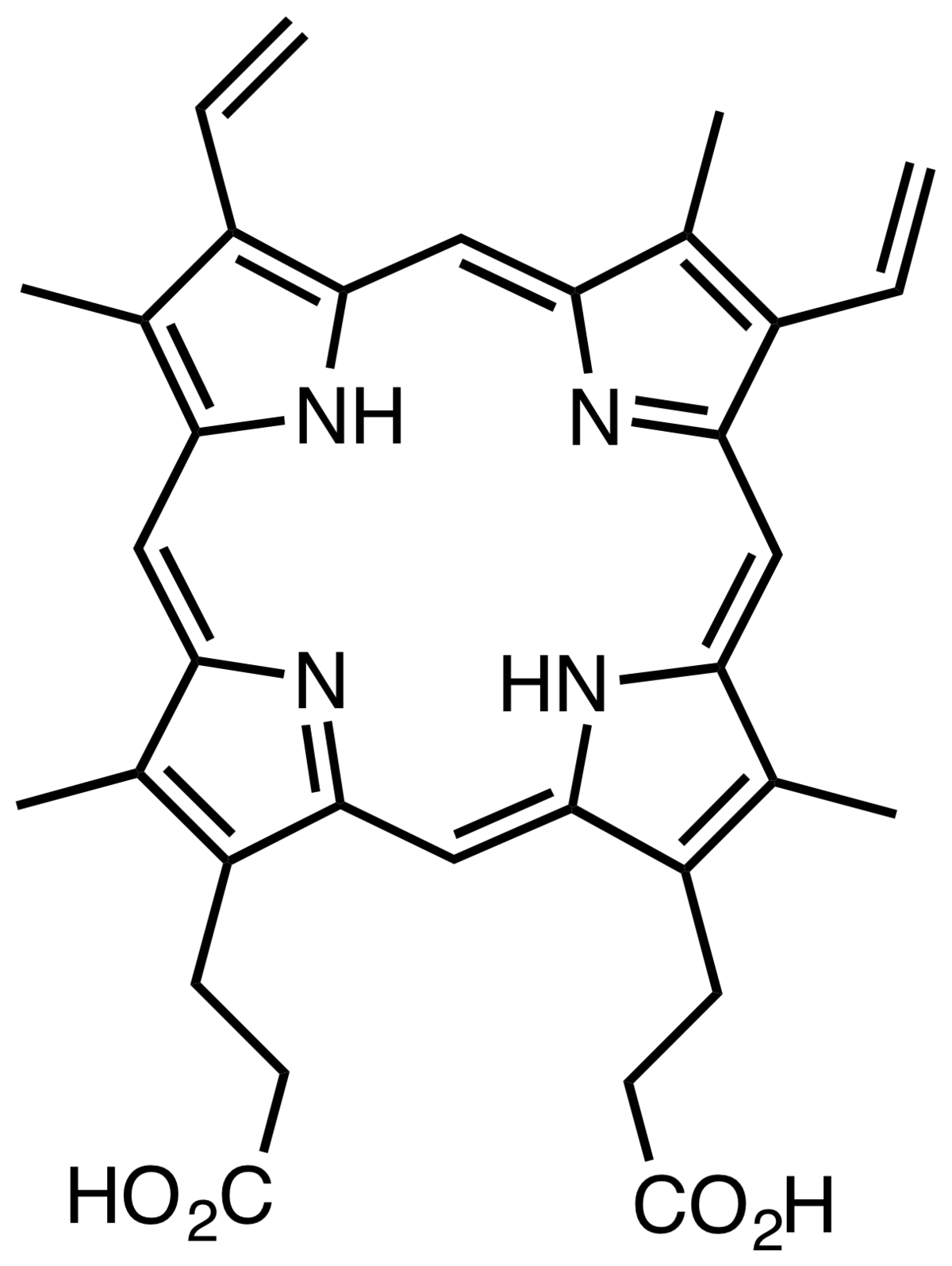
Where does synthesis of protoporphyrins occur? And what mediates it?
Occurs in the mitochondria of red cell precursors-mediated by EPO (Erythropoietin) and vitamin B6
-
What 2 components 'make' haem
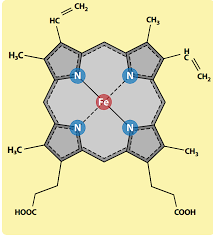
Protoporphyrin + Iron =haem
-
Where does globin synthesis occur?
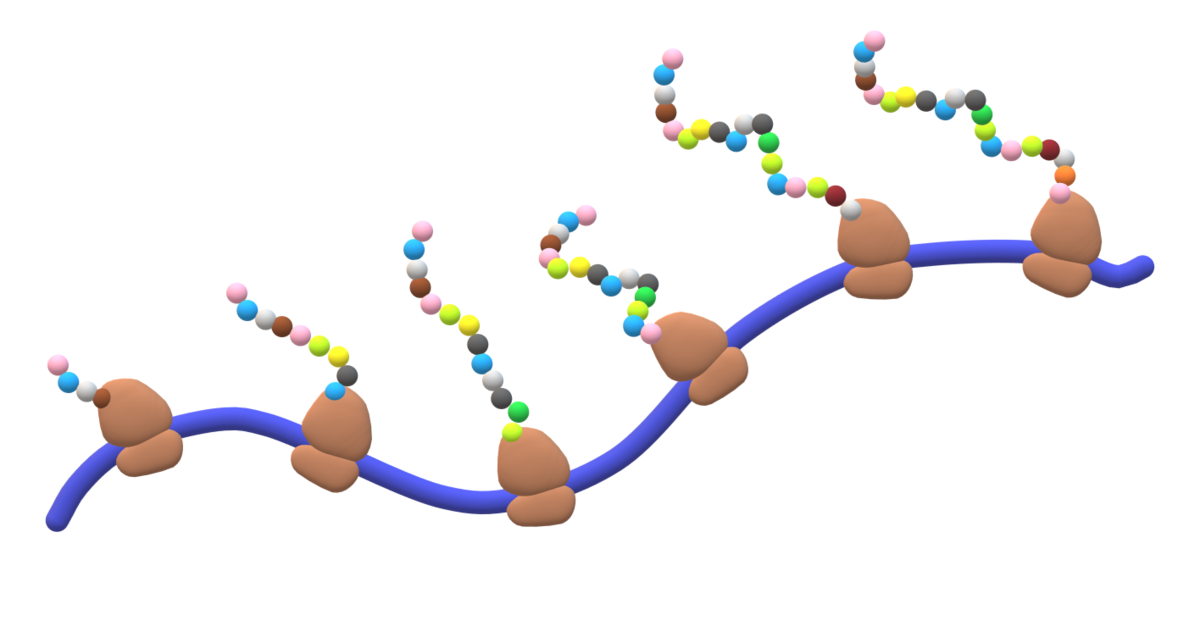
Occurs in the polyribosomes (RER)
+Rates of haem and globin synthesis are carefully coordinated to ensure optimal efficiency of Hb assembly
-
Proper globin synthesis depends on what?
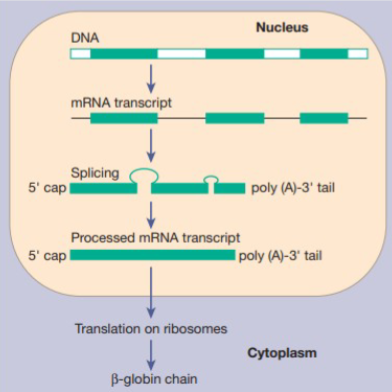
The genes, as precise order of amino acids in the globin chains is critical to the structure and function of haemoglobin
-
Statement: Various types of globin combine with haem to form different haemoglobin
Is this statement true or false?
TRUE
-
How many functional globin genes are there?
8
-
These globin genes are arranged in how many gene clusters?
2 duplicate gene clusters
-
State some information about these globin genes and clusters
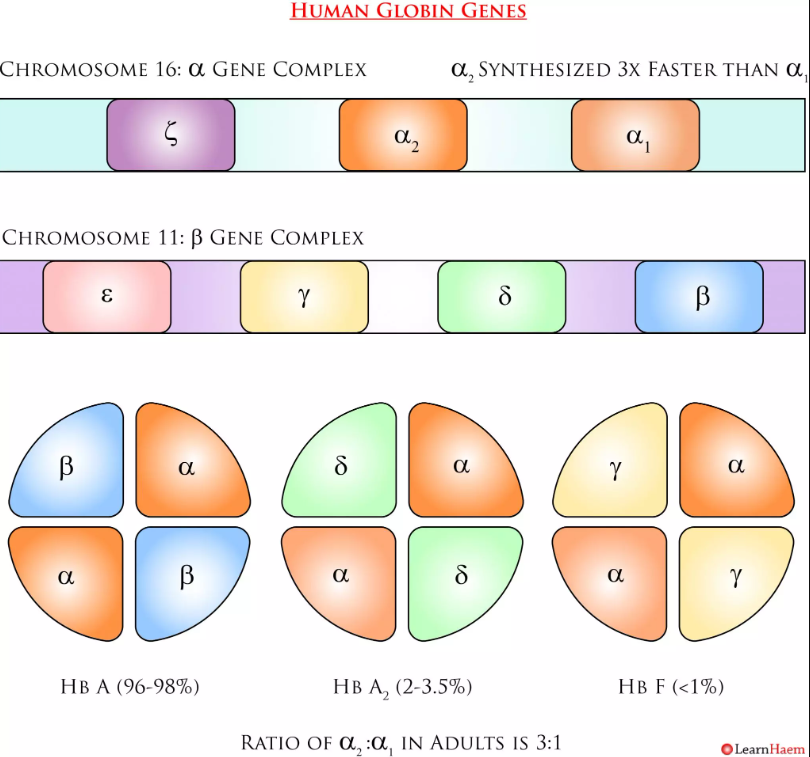
-These genes code for 6 different types of globin chains- β-cluster (β, γ, δ and ε globin chain) short arm ofchromosome 11
- α-cluster (α and ζ globin chain) short arm of chromosome 16
-
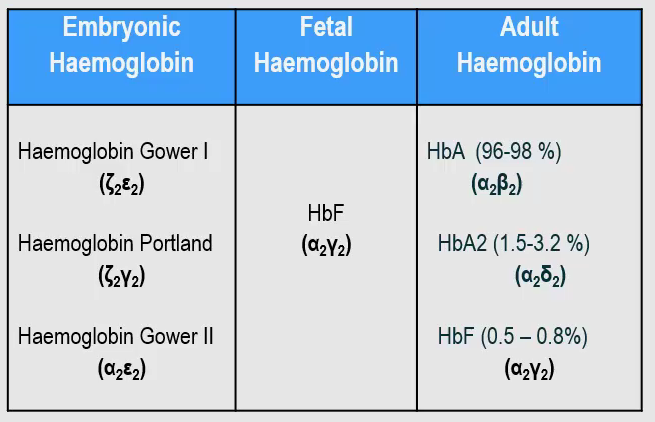
Picture demonstrating the varying amounts of globin synthesis in prenatal and postnatal life
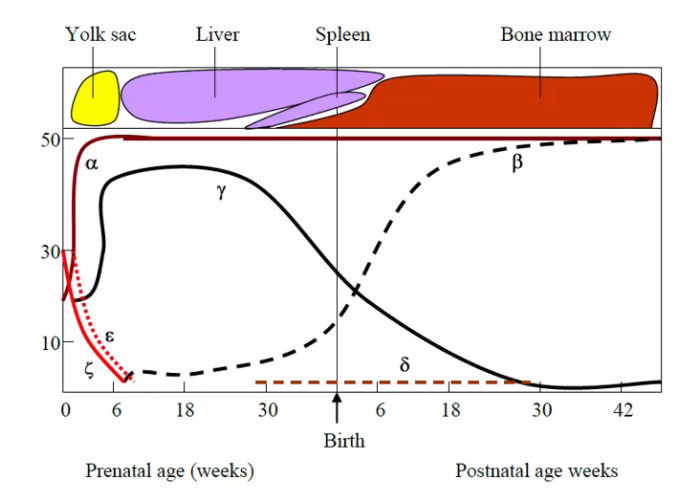
-
Name the 3 types of haemoglobin and their subtypes
-
FILL IN THE BLANKS:
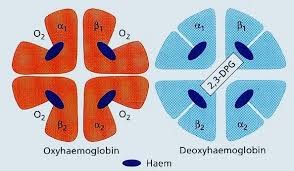
Red cells carry _________ from the lungs to the tissues;-Returns in _______ _______ with _____ from tissue to lungs-Buffering action, maintains ________ as it changes fromoxyhaemoglobin (carrying O2) to deoxyhaemoglobin (without O2)-One Hb in red cell can bind to ____ O2 molecules-Less than___ sec required for ____________.-When oxygenated ________ is pushed out, the ________ move closer.-When O2 is unloaded, ß-chains are _______ ______ to _________ _______of 2,3-BPG, resulting in lower affinity of Hb for O2
Red cells carry oxygen from the lungs to the tissues;-Returns in venous blood with CO2 from tissue to lungs-Buffering action, maintains blood pH as it changes fromoxyhaemoglobin (carrying O2) to deoxyhaemoglobin (without O2)-One Hb in red cell can bind to four O2 molecules-Less than .01 sec required for oxygenation.-When oxygenated 2,3-BPG is pushed out, the ß-chains move closer.-When O2 is unloaded, ß-chains are pulled apart to permit entry of 2,3-BPG, resulting in lower affinity of Hb for O2
-
The amount of O2 bound to haemoglobin and released to tissues depends on what?
-PO2 (partial pressure of oxygen)-PCO2, but also the-affinity of haemoglobin for O2.(P50 is the partial pressure of O2 at which Hb is 50% saturated)
-
What is oxygen affinity?
Oxygen affinity is the ease with which haemoglobinbinds and releases oxygen
-
What does oxygen affinity determine?
Determines the proportion of O2 released to the tissues or loaded onto the cell at a given oxygen pressure
-
What does an increase/decrease in oxygen affinity mean?
-Increase in oxygen affinity means haemoglobin has an increased affinity for O2, so it binds more & strongly to the O2.-Decreases in oxygen affinity, cause O2 to be released
-
The change in oxygen affinity with pH is known as the what?
The Bohr effect
-
Hb-O2 affinity is reduced as what increases?
Acidity
-
In acidic pH, the curve shifts to the left, resulting in an enhanced capacity to release O2 whereneeded
Is this TRUE?
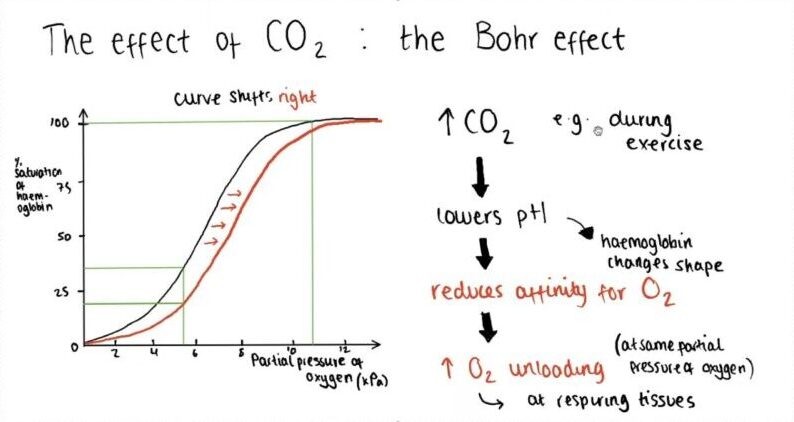
FALSE
In acidic pH, the curve shifts to the right, resulting in an enhanced capacity to release O2 whereneeded
-
Why is the pH lower in arterial blood?
-Since tissues are relatively rich in CO2, the pH is lower than in arterial blood;-So, the Bohr effect facilitates transfer of O2
-
The normal position of the Hb-Oxygen dissociation curve depends on what 4 factors?
-Concentration of 2,3-DPG-H+ ion concentration (pH)-CO2 in red blood cells-Structure of Hb
-
What are the standard conditions for the 'normal' positioning of the curve?
-Temp = 37OC-pH = 7.40-BE = 0
-
Changes in blood pH shift the Hb-O2 dissociationcurve.... why?
-Due to an Increased CO2 production by tissue & released into blood-Results in generation of H+ ions and decreased pH
-
What are the three mechanisms of transport (CO2)
-Dissolution in the plasma-Formation of Carbonic acid-Binding to form carbaminohaemoglobin
-
What are the five Haemoglobin derivatives?
-Oxyhaemoglobin (oxyHb): Hb with O2.
-Deoxyhaemoglobin (deoxyHb) = Hb without O2
-Methaemoglobin (metHb) - contains Fe3+
-Carboxyhaemoglobin (HbCO) - CO binds to Fe2+ in haem, in cases of CO poisoning
-Carbaminohaemoglobin (HbCO2) – CO2 binds non-covalently to globin chain of Hb. HbCO2 transports CO2 in blood
-
Inherited disorders of Hb can be split into two categories.....what are these 2 categories?
-The Thalassaemias
-Sickle cell disease
-
Mutations or deletions in the globin clusters 11 and 16 may lead to;
a) Abnormal synthesis of globin chain, as in Sickle Cell Diseases.b) Reduced rate of synthesis of normal globin chains, as in Thalassaemia.
-
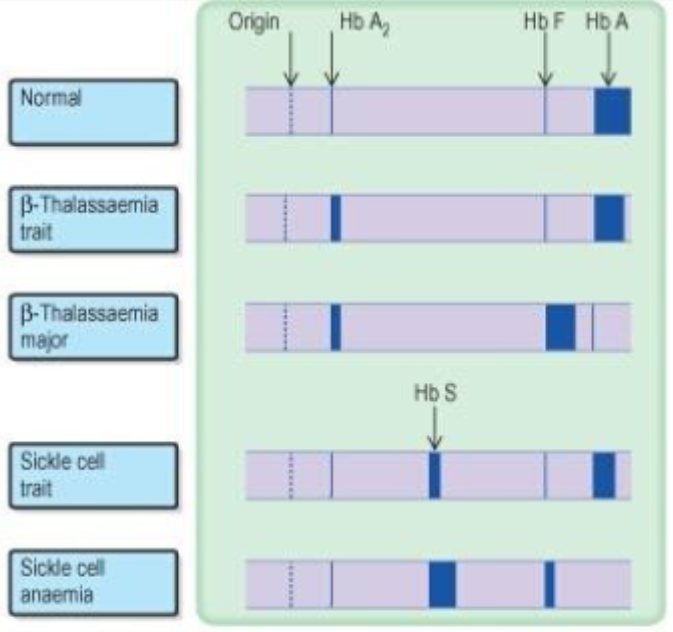
State some information on Sickle cell disease
Sickle Cell Disease-Group of Hb disorders with inherited sickle -globin gene.-Sickle Cell Anaemia (HbSS), homozygous, most common;
Normal β-globin = CCT GAG GAGSickle β-globin = CCT GTG GAG-heterozygote conditions: HbS/ßthal, HbSC, HbSD-Treatment: aim to induce HbF
-
State some information on the Thalassaemias
Beta-thalassaemia
-Loss of 1 b-chain causes mild microcytic anaemia (thalassaemia trait)
-Loss of both (b^0) causes thalassaemia major
-Excess α-chains precipitate in erythroblasts, causing haemolysis and ineffective erythropoiesis.
Alpha-thalassaemia
-There can be loss of 1, 2, 3 or 4 alpha chains.
-
(Blurry) Picture demonstrating haemoglobin electrophoresis