-
Atoms
➤ smallest and simplest form of matter that gives uniqueness to elements.
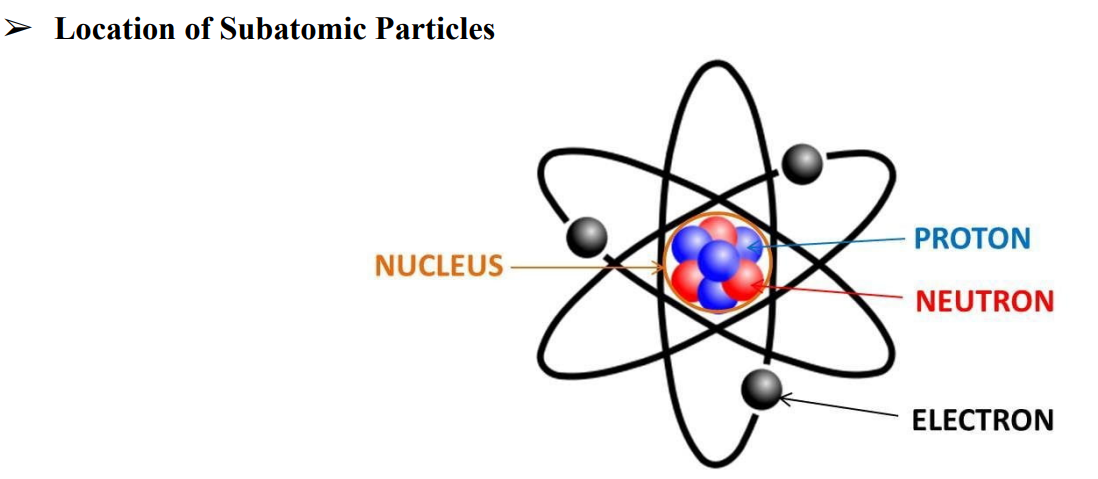
➤ Inside the central nucleus are the subatomic particles
-
Molecules
combination of two or more atoms
-
Particles
general term (moving)
-
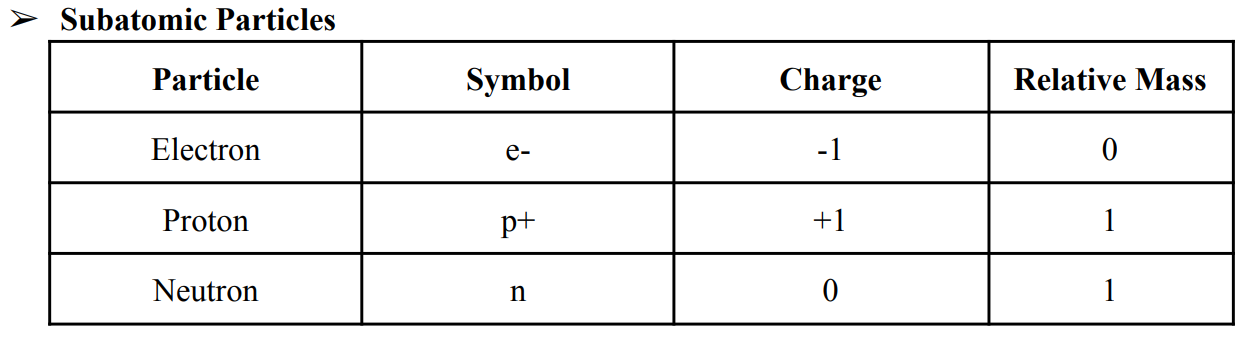
Subatomic Particles
protons, neutrons, and electrons
-
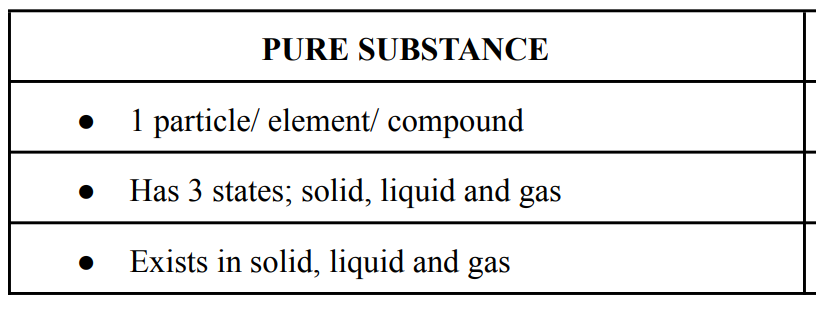
Matter
anything that has mass & volume and that occupies space.
-
Elements
are composed of one type of atom.
-
-
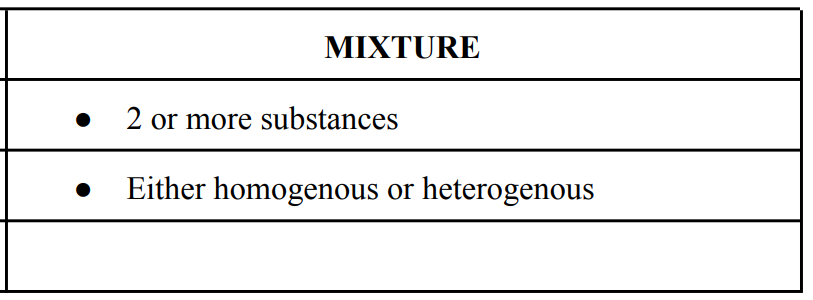
Types of Mixtures
Heterogenous
can see the different parts of the mixture easily/ visible.
-
Types of Mixtures
Homogenous
can see the different parts of the mixture easily/ visible.
-
SCIENTIFIC METHOD (7)
1. Identify the problem.
2. Gather enough information.
3. Formulate Hypothesis.
4. Test the hypothesis.
5. Recording and Analyzing Data.
6. Make a Conclusion.
7. Verification of Conclusion.
-
Compound
A pure substance consisting of two or more elements with a definite composition, that can be broken down into simpler substances only by chemical methods
-
States of Matter
Three forms of matter: solid, liquid, and gas
-
Atomic Mass
The weighted average mass of all the naturally occurring isotopes of an element
-
PRINCIPLES OF MATTER
1. All matter is made up of tiny particles.
2. There is empty space between the particles.
3. The particles are in constant motion.
4. There are forces that act between the particles
-
Solid
have a definite shape and volume
-
Liquid
have a definite volume, but take the shape of the container.
-
Gas
have no definite shape or volume.
-
Physical Properties
are the appearances of an object, they can vary in size, shape, texture, color or mass.
-
Intensive Property
independent of the amount of materials; can be measured.
-
Extensive Property
not independent (texture, color) and no measurement.
-
Physical change
change in the appearance of one matter (shape, size or state but not composition).
-
Chemical Properties
the chemical composition of an object.
-
Chemical change
reaction or change of the composition of a matter with another substance.
-
Atomic Theory
➤ Atoms are building blocks of elements.
➤ Similar atoms are in each element
➤ 2 or more bond to create compound with the ratio ( 1 : 2 : 1)
-
-
Atomic Symbols
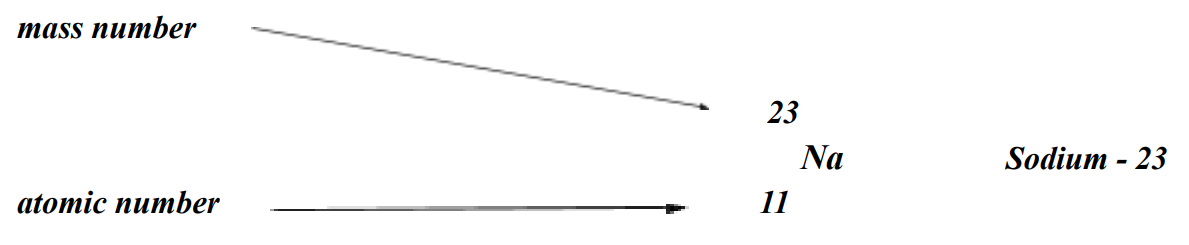
show the mass number and atomic number
-
Isotopes
are atoms that have the same atomic number, protons or elements but different mass numbers and neutrons.
-
Atomic Mass
the weight average mass of all the atomic masses of the isotopes of the atom. It is the result of when you round off the atomic weight^2 in your periodic table.
Example of an average atomic mass:
Chlorine: Cl-35 is about 75.5% and Cl-37 is 24.5% of natural chlorine.
35 × 75.5/ 100 = 26.4
37 × 24.5/ 100 = 9.07
-
Mass numbers
are the counts of protons and neutrons.
-
Formula
Mass Number
◆ Mass number (MN) = p+ (protons) + n (neutrons)
-
Formula
Neutrons
◆ n (neutron) = MN (mass number) - p+ (protons)
-
Formula
Protons
◆ p+ (protons) = the atomic number of an element
-
Formula
Electrons
◆ e- (electrons) = p+ (protons) - charge
-
Number of Electrons
An atom is neutral
-
Number of Electrons
If the atoms are neutral = net charge is zero = number of protons is equal to electrons.
-
Number of Electrons
Atomic number will be equal to the number of electrons if neutral.
-
Number of Electrons (3)
➤ An atom is neutral.
➤ If the atoms are neutral = net charge is zero = number of protons is equal to electrons.
➤ Atomic number will be equal to the number of electrons if neutral.
-
Measurement
➤ Is a quantitative observation
➤ Is the process of getting the actual measure of an object dimension's property in comparison with a standard unit of acquiring a value.
➤ Two parts: number and scale (unit)
-
Significant Figures
include digits which are certain + 1 uncertain digit.
-
Nonzero Integers
always count as significant figures.
-
Leading Zeros
zeroes that precede all nonzero digits; don’t count as significant figures; only function is to know the decimal position/place of a number.
● Ex: 0.0025 > only 2 and 5 is the significant numbers
-
Captive Zeros
zeroes between nonzero digits; counts as SF.
● Ex: 1.008 > we have four significant figures
-
Trailing Zeros
zeroes right end of the number; significant only if the number has a decimal point.
● Ex: In 100 > only 1 is the SF, but if it is written as 100. then we’ll have three SF.
● Another example is in 1.00 x 10^2 > we’ll have three SF
-
Exact Numbers (3)
◆ Not obtained using measuring devices but by counting; example 10 experiments or 3 apples.
◆ Can be assumed to have infinite numbers of significant figures; example is the circumference of a circle or the volume of a sphere.
◆ Can also arise from definitions; example 1 inch is defined by 2.54 cm, neither of the numbers are the limit of significant figures when calculated.
-
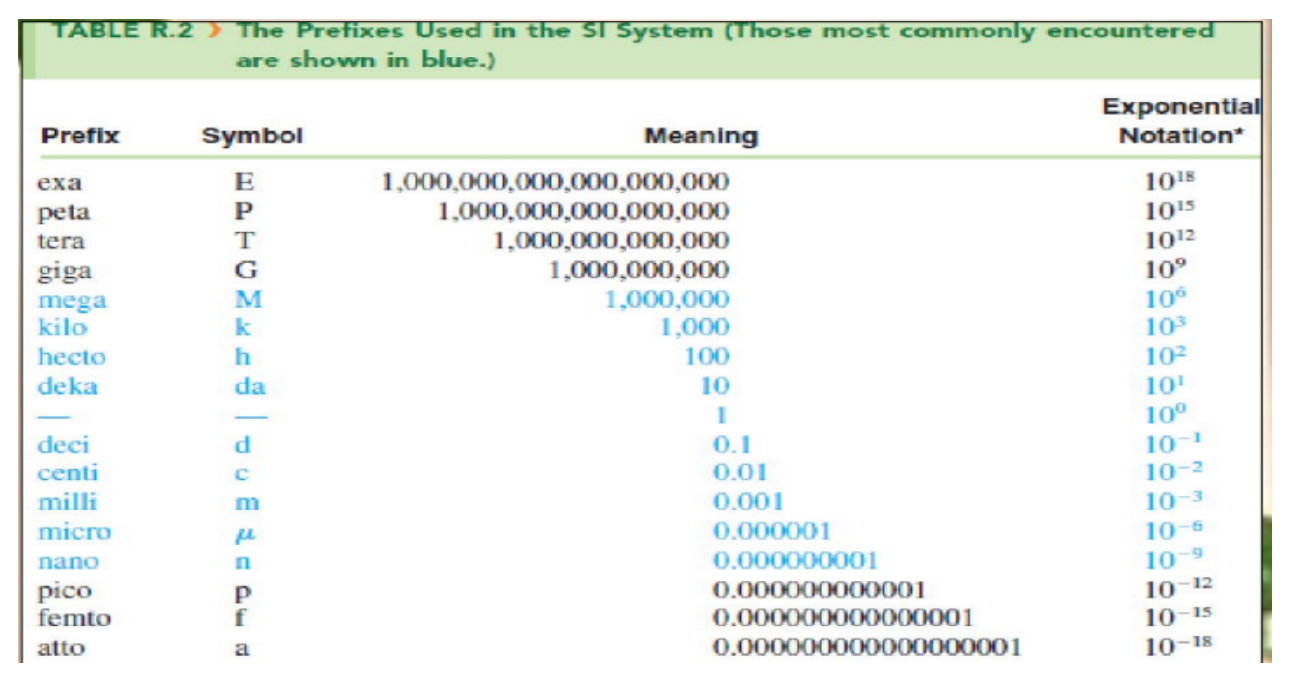
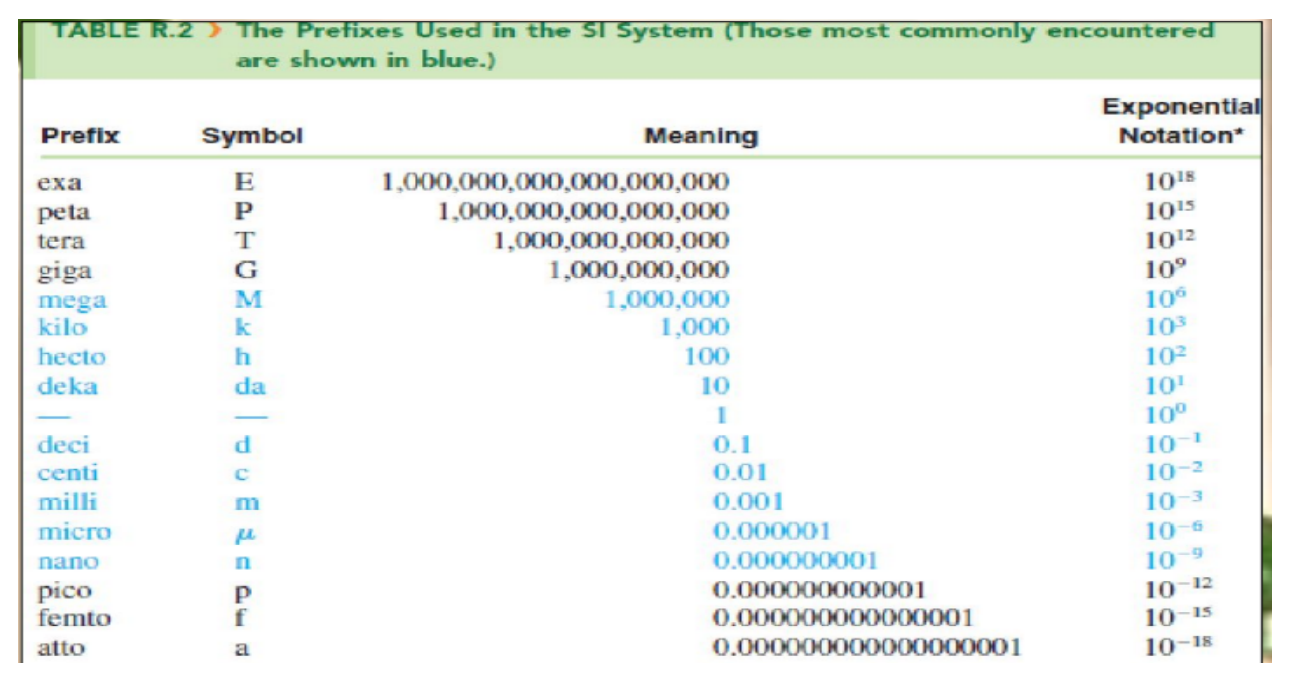
English System
the British system of measurement was first adopted in England and later became widely used in the United States; also known as the FPS system, derived from the names of the standard units of length (foot), weight (pound), and time (second)
-
English System
this system also include the inch, yard, mile, and horsepower
-
Metric System
originated in France in 1791; has the units of meter, centimeter, kilogram, and second
-
Metric System
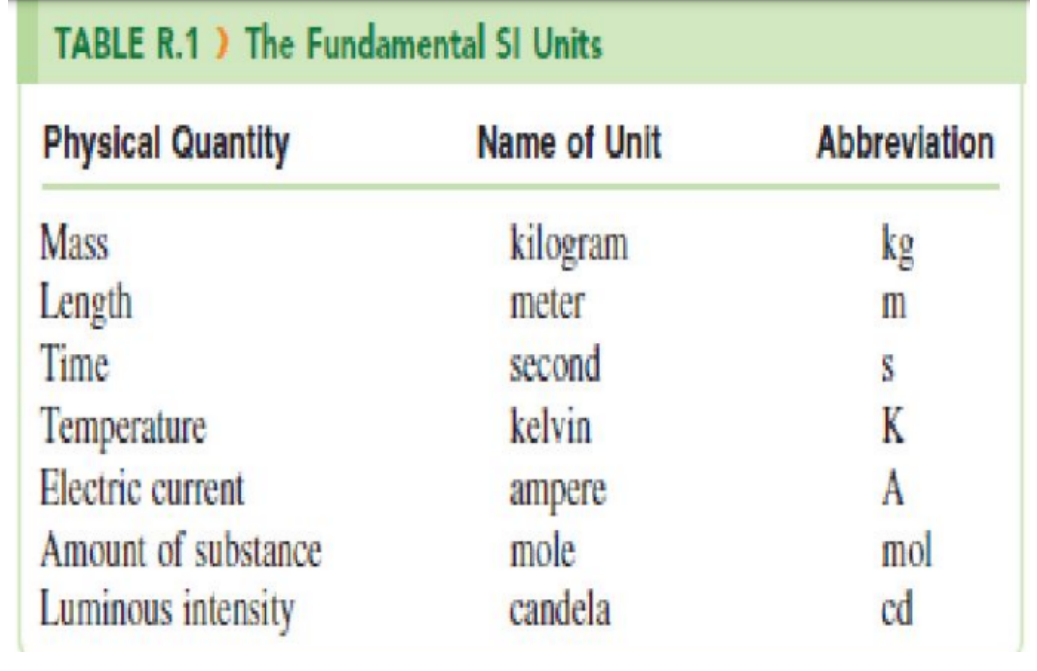
the commonly used method of measurement the modern metric system has been officially named and known worldwide as the International System of Units
-
-
BASIC TYPES OF QUANTITY:
1. Fundamental Quantities
are the fundamental units like meters, kilograms and seconds and are also the standard units for length and time, respectively.
- However, for smaller quantities, centimeters, grams and seconds are used as fundamental units.
-
BASIC TYPES OF QUANTITY:
2. Derived Quantities
these are quantities that emanate or are a result of the combination of fundamental quantities after a set of operations.
-
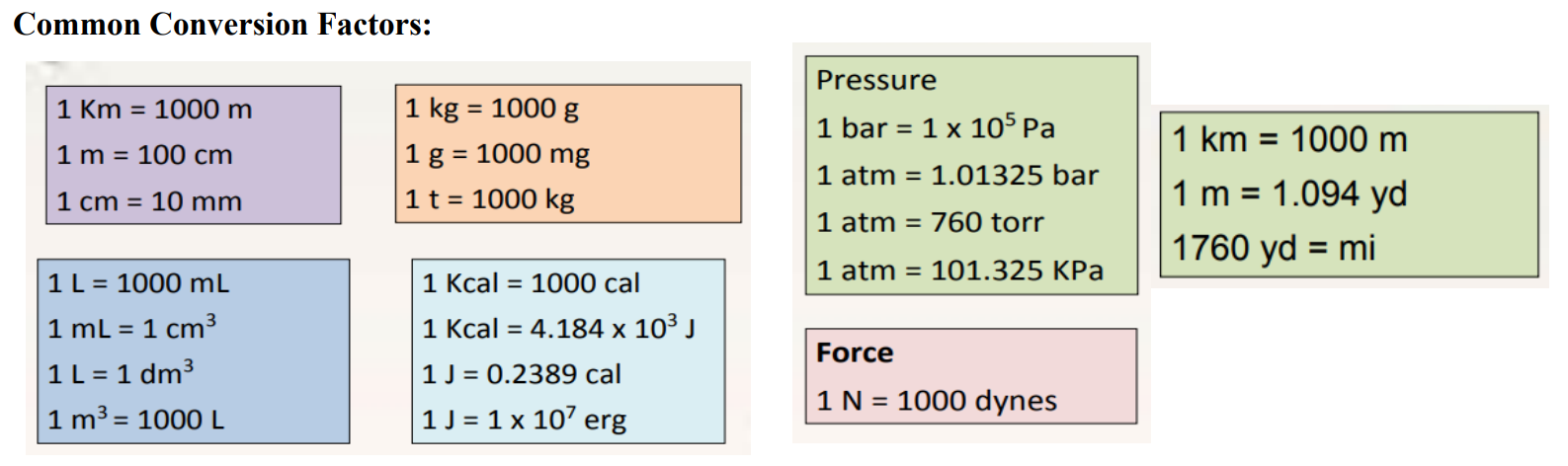
Conversion of Units (Formula):
Given unit × Desired unit = Desired unit
Given unit
-
Scientific Notation
A number in scientific notation has two parts: the first is a number between 1 and 10 (N), and the second part is a power of 10 (N^10).
-
Periodic Table
chart in which elements having similar chemical and physical properties are grouped together.
○ Period = horizontal
○ Groups/ Families = vertical
-
Metal
good conductor of heat and energy; positively charged.
-
Non-metal
poor conductor of heat and energy.
-
Metalloid
has properties that are intermediate between those of metals and non-metals.
-
Alkali Metals
Group 1A elements ( Li, Na, K, Rb, Cs and Fr)
-
Alkaline Earth Metals
Group 2A elements (Be, Mg, Ca, Sr, Ba and Ra)
-
Halogens
Group 7A (F, Cl, Br and At)
-
Noble Gases/Rare Gases
Group 8A (He, Ne, Ar, Kr, Xe and Rn
-
Static Electricity
happens when we have electrical discharge
○ Example: Getting grounded after friction on a carpet then holding onto a metal thing.
○ A short circuit can happen after getting electrified and that causes death (Example is getting struck by lightning).
-
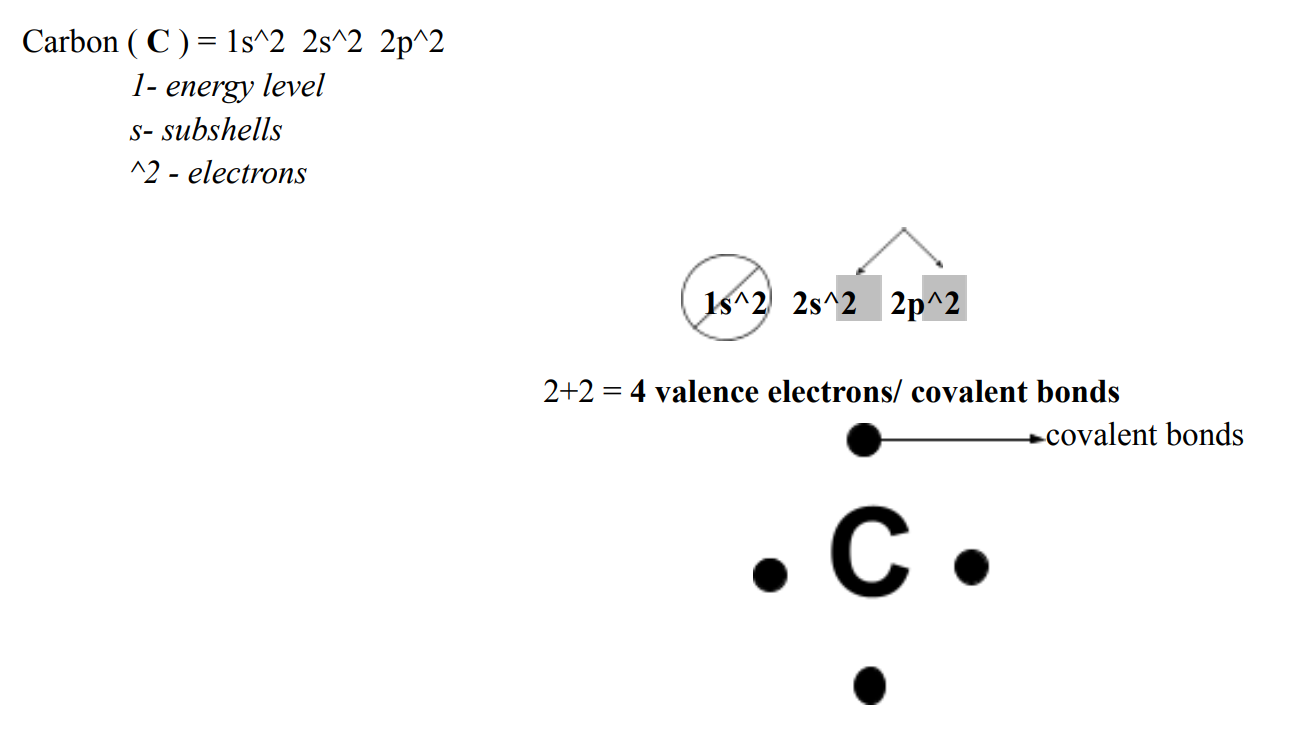
Molecule
2 or more atom bonded chemically
○ Diatomic Molecule = only 2 atoms
○ Polyatomic Molecule = more than 2 atoms
-
Ion
a group of atoms that has net positive or negative charge.
-
Cation
ion with a positive charge
-
Anion
ion that has a negative charge because of an increase in the electrons.
-
Monoatomic Ions
only 1 atom
-
Polyatomic Ion
more than 1 atom
-
Chemical Formulas
expresses composition of molecules and ionic compounds in terms of chemical symbols.
-
Molecular Formula
exact numbers of atoms of each element in the smallest unit of a substance.
■ All tropes, one of 2 or more distinct forms; can be affected by light, temperature and pressure.
-
Structural Formula
how atoms in a molecule are bonded to one another.
-
Molecular Formula
used to visualize
-
Ball-and-Stick Models - ball = atoms, connected by sticks = chemical bonds.
Space-Filling Models - atoms = by overlapping spheres, proportional in size to the corresponding atoms; no bonding and mostly emphasizes on molecule size.
-
Empirical Formula
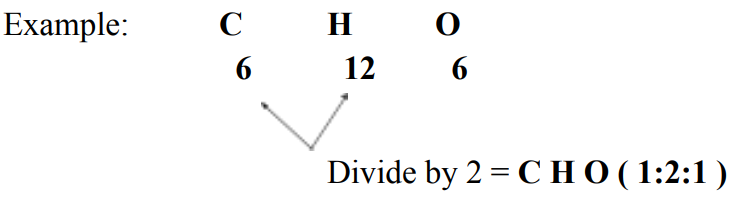
Empirical Formula - always in proportion or ratio; written by reducing subscripts in molecular formulas to the smallest possible whole numbers.
-
Ionic Compounds
compounds formed from cations and anions
-
Formulas for Ionic Compounds
Formulas for Ionic Compounds are usually the same as their empirical formulas because they do not consist of discrete molecular units. For the formula to be electrically neutral, the sum of the charges on the cation and anion in each formula must be ZERO.
-
If the magnitudes of the charges are not zero, make it neutral by having the subscript of the cation numerically equal to the charge on the anion, and the subscript of anion will be numerically equal to the cation.

-
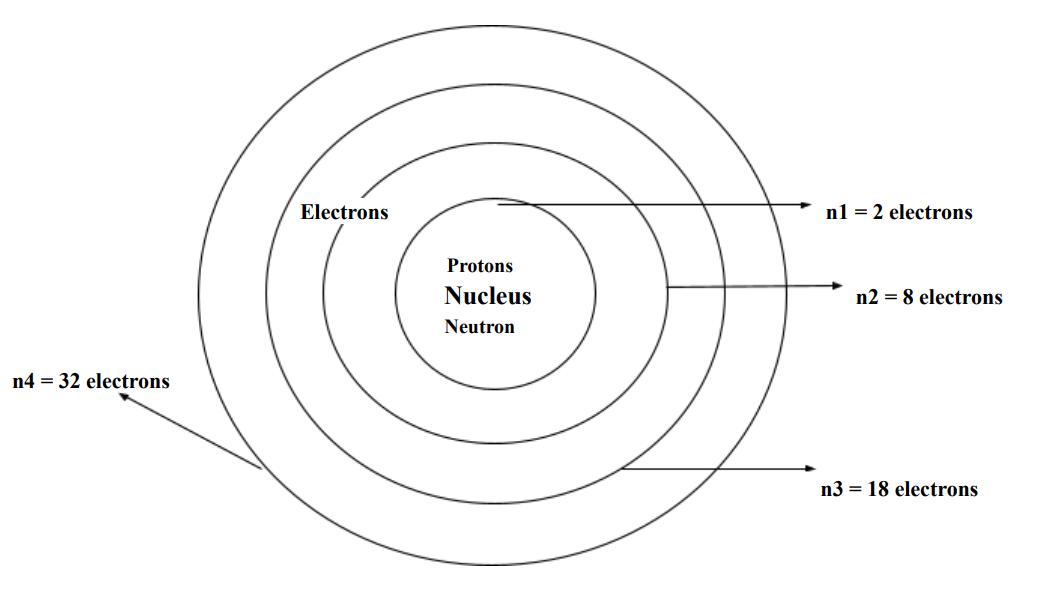
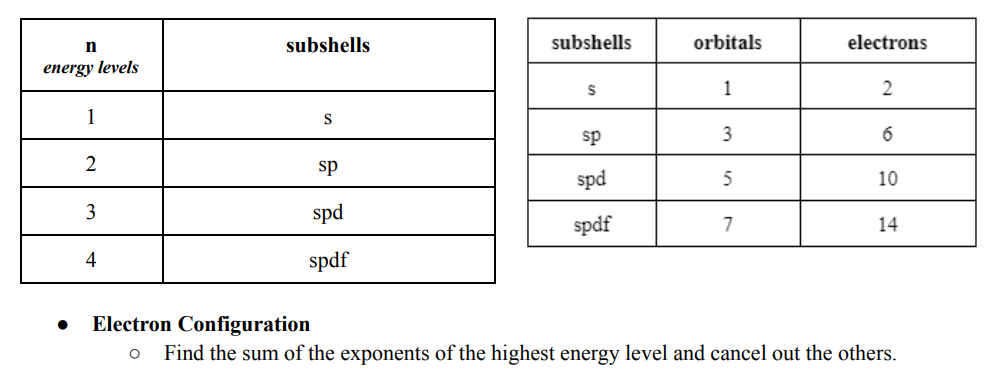
● n (energy levels/ shells) = principle of quantum numbers
Formula: 2(n)^2
n2 = 2(2)^2 = 8 electrons
Shells hold only specific numbers of electrons.
● Orbitals can also hold a certain number of electrons.
○ Electrons in orbitals = n x 2
-