Control of Blood Glucose & The Endocrine Pancreas (Physiology)
Control of blood glucose & the endocrine pancreas - Dr Peter Skorupski Session Summary The hormones of the endocrine pancreas, insulin and glucagon, are central to maintaining blood glucose homeostasis. Both hormones are exquisitely sensitive to plasma glucose concentration, with the ratio of insulin to glucagon increasing (in favour of insulin) as glucose concentration rises, and decreasing (in favour of glucagon) as the glucose concentration falls. However, there are many additional factors affecting secretion, including other nutrients, other hormones, and neural influences. Overall these serve to maintain appropriate hormonal responses across a variety of nutritional states. We will review the secretion and release of insulin and glucagon in response to changes in plasma glucose and other factors, as well as their receptor-mediated effects. We will define type 1 and type 2 diabetes mellitus and touch on the difficulties in maintaining glycaemic control in these diseases. Learning outcomes At the end of this session you will be able to: Describe the transport of glucose across the cell membrane Identify the cells of the endocrine pancreas and describe the factors that control insulin and glucagon secretion Identify the receptors for insulin and glucagon and outline their mode of action Define type 1 and type 2 diabetes mellitus Explain the incretin effect
-
How does glucose enter cells? (2)
☆Glucose enters cells through sodium-glucose cotransporters (SGLTs), which utilize secondary active transport mechanisms.
☆SGLT1 is involved in glucose absorption from the gut, while both SGLT1 and SGLT2 facilitate glucose reabsorption from the kidney (proximal convoluted tubule).
-
How does glucose enter cells? (7)
☆Glucose enters cells through a family of glucose transporters (GLUTs).
☆Different GLUT isoforms have varying affinities for glucose and are expressed in different tissues.
☆GLUT1 is found in the brain and erythrocytes.
☆GLUT2 is present in the liver, kidney, pancreas, and gut.
☆GLUT3 is predominantly expressed in the brain.
☆GLUT4 is found in muscle and adipose tissue.
☆Insulin stimulates the translocation of GLUT4 transporters to the cell membrane, facilitating insulin-dependent uptake of glucose into cells.
-
What are the hormones of the endocrine pancreas (2)
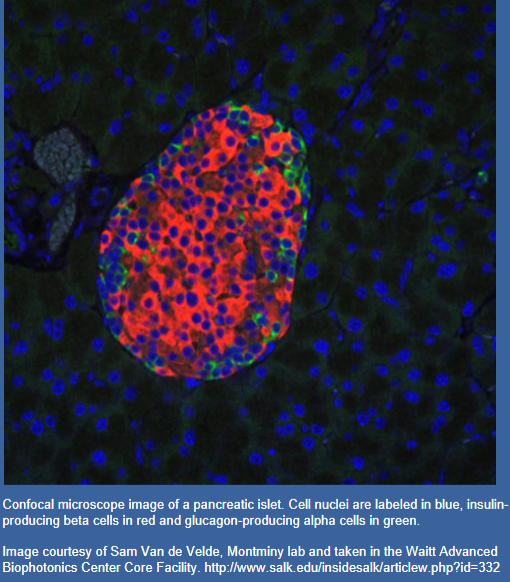
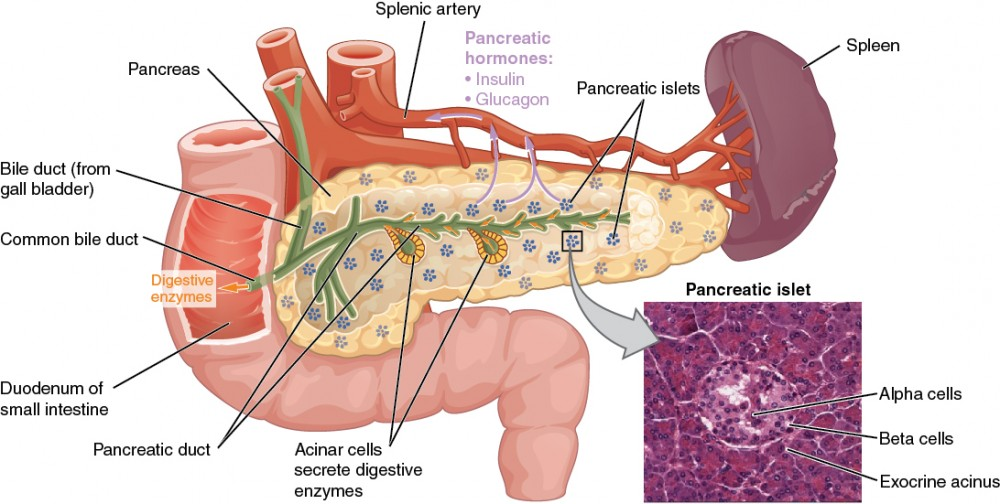
☆The islets of Langerhans, located within the endocrine pancreas, consists of different types of hormone-secreting cells.
☆These include α-cells (A cells), which produce glucagon; β-cells (B cells), responsible for insulin secretion; and δ-cells, which produce somatostatin.
-
How is insulin synthesised post transcription? (5)
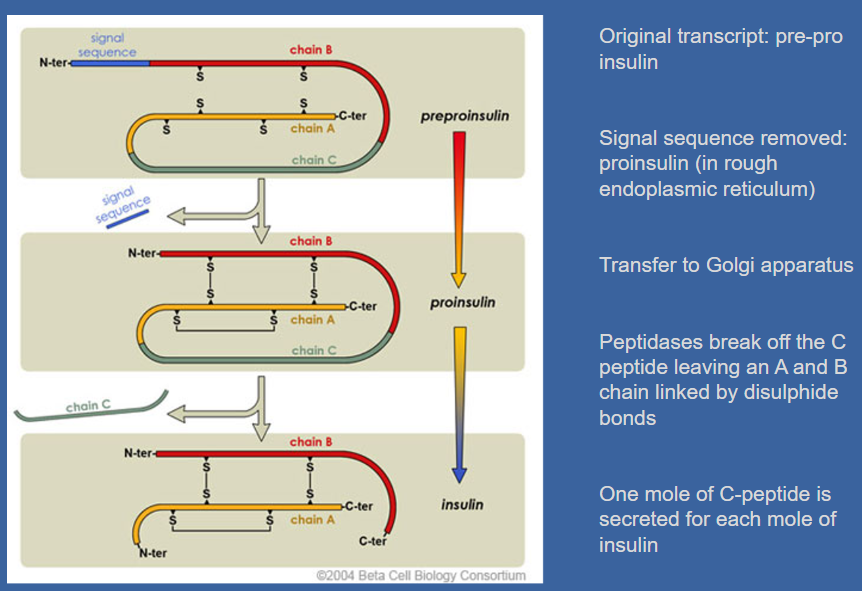
☆Insulin synthesis begins with the production of pre-proinsulin, which contains a signal sequence.
☆This sequence is removed, forming proinsulin in the rough endoplasmic reticulum.
☆Proinsulin is then transferred to the Golgi apparatus. ☆Peptidases in the Golgi apparatus cleave off the C-peptide, leaving behind the A and B chains linked by disulphide bonds.
☆It's important to note that one mole of C-peptide is secreted for each mole of insulin.
-
How is insulin released into the circulation? (4)
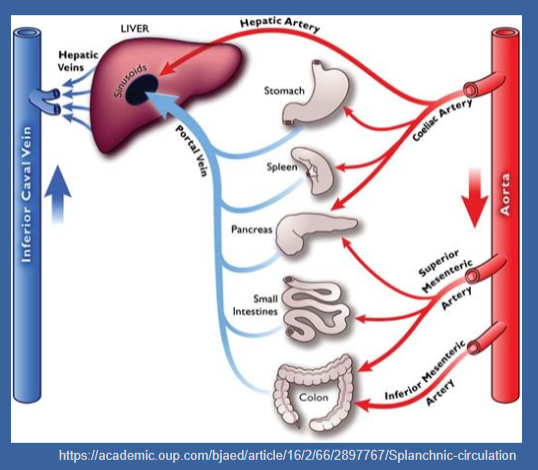
☆The pancreas receives its blood supply from branches of the coeliac, superior mesenteric, and splenic arteries.
☆The venous drainage of the pancreas is into the portal system.
☆Approximately, half of the secreted insulin undergoes metabolism by the liver during its first pass, while the remaining insulin is diluted in the peripheral circulation.
☆C-peptide serves as a more accurate index of insulin secretion in the peripheral circulation, as it is not metabolized by the liver.
-
Picture demonstrating factors that regulate insulin secretion:
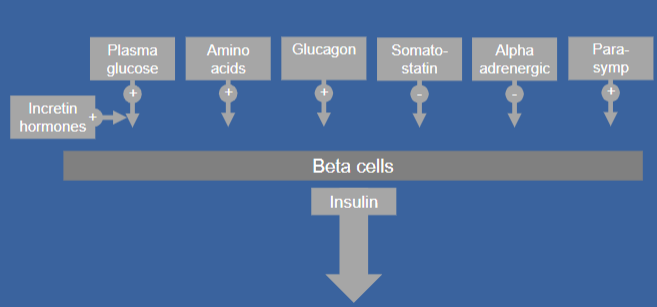
-
Picture demonstrating factors that regulate glucagon secretion:
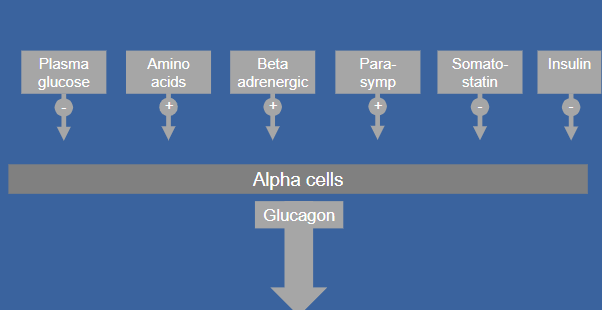
-
Picture of Insulin and glucagon secretion are exquisitely sensitive to blood glucose levels
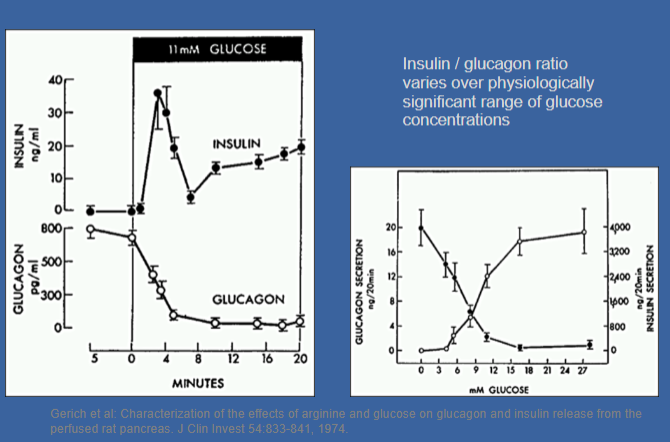
-
How do β cells sense a rise in glucose? (4)
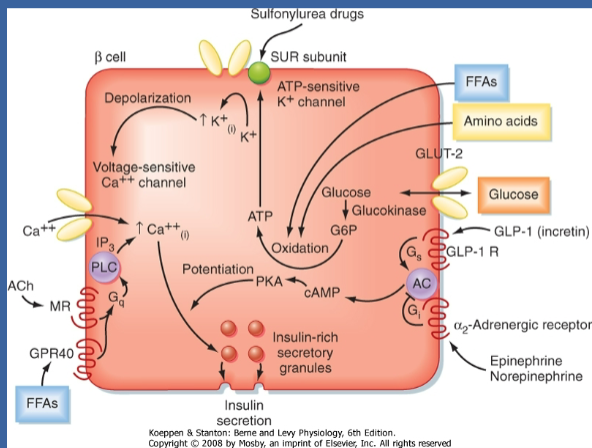
☆β cells lack a specific glucose receptor.
☆Instead, GLUT2 and glucokinase serve as sensors.
☆When glucose enters the β cell via GLUT2, it undergoes phosphorylation by glucokinase (initiates the krebs cycle).
☆This process leads to an increase in ATP levels due to glucose oxidation, serving as the effector for sensing the rise in glucose.
-
How does insulin binding to its receptor affect cellular processes? (4)
☆When insulin binds to its receptor, a member of the tyrosine kinase superfamily, it initiates several protein activation cascades.
☆These cascades include the translocation of the Glut-4 transporter to the plasma membrane, leading to the influx of glucose.
☆Additionally, insulin signalling stimulates glycogen synthesis, glycolysis, and fatty acid synthesis.
☆This binding activates a cascade of protein phosphorylation, which modulates the activity of specific metabolic enzymes by regulating enzyme phosphorylation and gene transcription.
-
Picture demonstrating insulin receptor action:
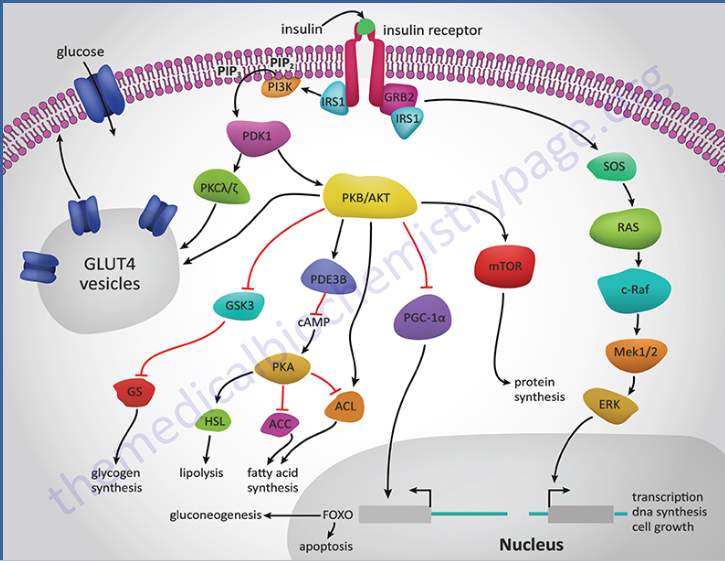
-
Picture demonstrating glucagon receptor action:
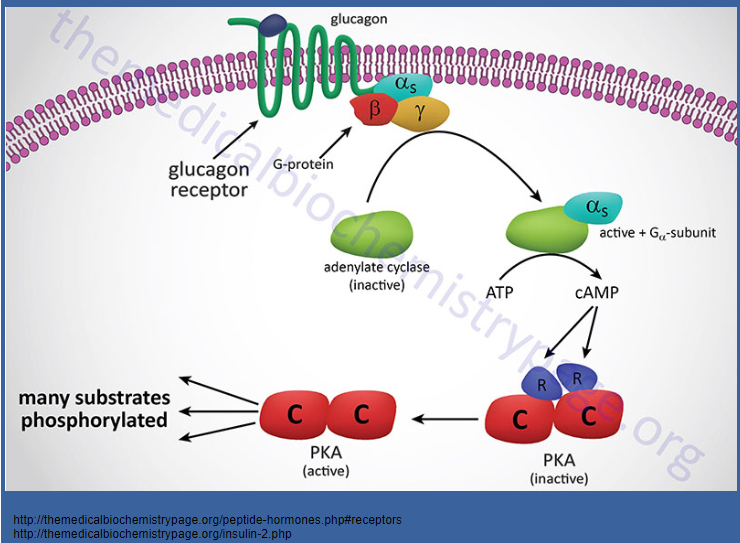
-
How do insulin and glucagon exert their opposing effects on metabolic pathways? (3)
☆Insulin and glucagon, as counter-regulatory hormones, primarily regulate metabolic pathways through the activity of protein kinase A (PKA).
☆Glucagon activates PKA, leading to the phosphorylation of key enzymes involved in metabolic pathways.
☆In contrast, insulin action results in the dephosphorylation of these same enzymes, exerting an opposing effect to glucagon.
-
What is the historical significance of diabetes mellitus? (1)
☆Known since ancient times, diabetes mellitus was invariably fatal until the availability of insulin extracted from animal pancreas in 1922.
-
Diabetes and the pancreas: timeline (5 no harsh marking)
☆1869: Paul Langerhans describes “islands of clear cells” in his thesis on the microanatomy of the pancreas. Their function remains unknown at this time.
☆1890: Oskar Minkowski removes the pancreas from a dog in an experiment with von Meyer to study the role of pancreatic secretions in fat digestion. The dog subsequently develops diabetes, establishing the pancreatic origin of diabetes.
☆1921: Frederick Banting collaborates with Charles Best in the laboratory of JJR Macleod at the University of Toronto, conducting experiments on administering pancreatic extracts to diabetic dogs. Biochemist James Collip later joins the team.
☆1922: Pancreatic extract is administered to a 14-year-old boy suffering from diabetic ketoacidosis (DKA). The treatment results in a dramatic improvement, marking the first human life saved by insulin.
☆1923: Frederick Banting and JJR Macleod are awarded the Nobel Prize for the discovery of insulin.
-
Diabetes mellitus: classification (2)
☆Type 1 diabetes is characterized by absolute insulin deficiency, resulting from the destruction of insulin-producing pancreatic beta cells.
☆Type 2 diabetes involves a variable combination of insulin resistance and insulin insufficiency.
-
How is Hyperglycemia is central to diabetes mellitus diagnosis (3)
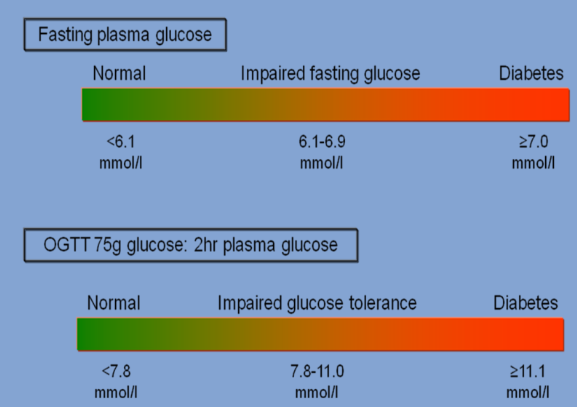
Diagnosis of diabetes mellitus is confirmed with the following criteria:
☆Random plasma glucose level ≥ 11.1 mmol/L.
☆Fasting plasma glucose level ≥ 7.0 mmol/L.
☆Oral glucose tolerance test (OGTT) result ≥ 11.1 mmol/L.
-
How does insulin contribute to the regulation of plasma glucose levels? (2)
☆Insulin plays a crucial role in maintaining stable plasma glucose levels despite variations in sugar intake and physical activity.
-
How does insulin help maintain stable plasma glucose levels despite variations in sugar intake and physical activity? (2)
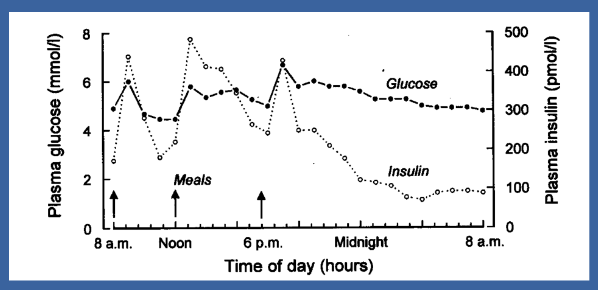
☆Insulin ensures stable plasma glucose levels by facilitating the uptake of glucose into cells, where it can be stored or used for energy production.
☆Additionally, insulin promotes the conversion of excess glucose into glycogen for storage in the liver and muscles, preventing hyperglycaemia.
-
Why is glycaemic control important in diabetes management? And how is it measured? (4)
☆Glycaemic control is crucial for reducing both macro- and microvascular complications associated with diabetes.
☆Monitoring glycosylated hemoglobin (A1C) levels serves as an indicator of glycaemic control, with levels below 6.5% considered optimal.
☆Achieving lower A1C levels can lead to significant reductions in the risk of microvascular complications, with every 1% decrease resulting in a 20-30% relative risk reduction.
☆However, maintaining optimal glycaemic control can be challenging.
-
What is the incretin effect and why is it significant in type 2 diabetes mellitus (T2DM)? (3)
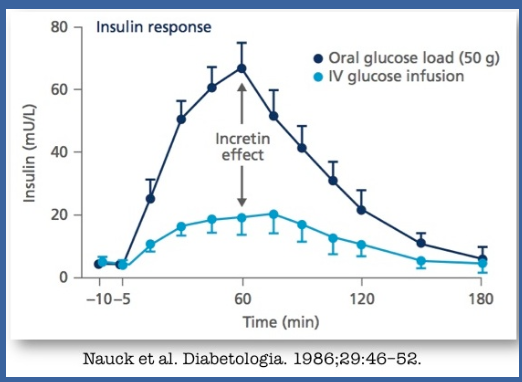
☆The incretin effect refers to the enhanced release of insulin from pancreatic beta cells in response to oral nutrient ingestion, particularly from the gut-derived incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).
☆There is evidence suggesting impairment of the incretin effect in individuals with T2DM.
☆Consequently, targeting the incretin system has become a major focus for the development of new drugs aimed at managing T2DM.
-
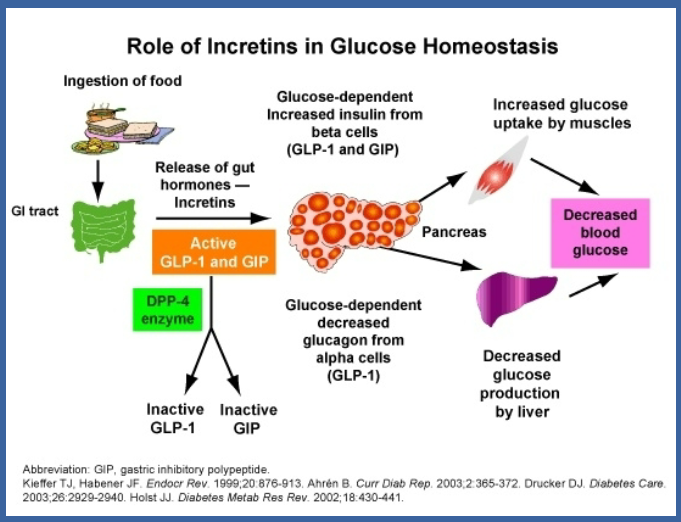
Picture demonstrating The incretins: glucagon-like peptide-1 (GLP-1) and GIP:
-
How does glucagon-like peptide-1 (GLP-1) receptor activation contribute to insulin release? (2)
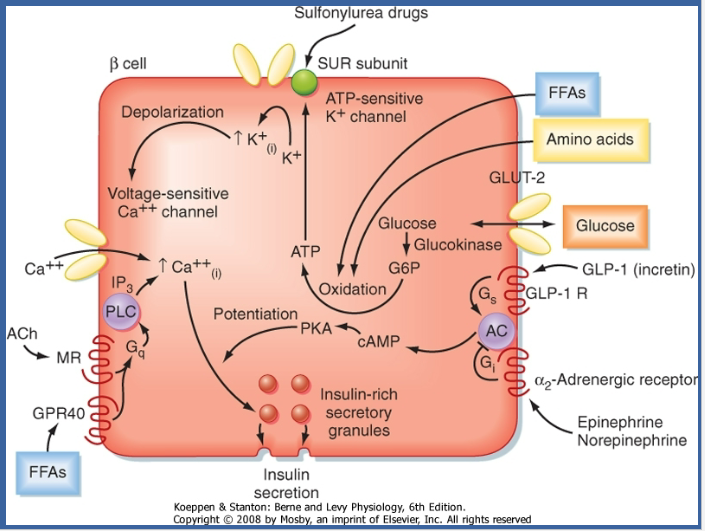
-
How does glucagon-like peptide-1 (GLP-1) receptor activation contribute to insulin release? (2)
☆Activation of the GLP-1 receptor leads to the activation of adenylyl cyclase, which increases intracellular levels of cyclic adenosine monophosphate (cAMP).
☆This amplifies glucose-induced insulin release from pancreatic beta cells, as increases in cAMP activate PKA
-
What significant changes did the discovery of insulin bring to the prognosis of Type 1 Diabetes Mellitus (T1DM)? (2)
☆The discovery of insulin transformed the prognosis of Type 1 Diabetes
☆Mellitus (T1DM) from an inevitable death due to diabetic ketoacidosis (DKA) to a manageable condition.
-
What challenges are associated with insulin therapy for achieving normal glycaemic control? (1)
☆Despite insulin therapy, normal glycaemic control is often not achieved in diabetes management.
-
What is the likely progression path of Type 2 Diabetes Mellitus (T2DM) in terms of insulin dependence? (1)
☆Type 2 Diabetes Mellitus (T2DM) likely begins with insulin resistance and may progress to full insulin dependence.
-
What factors contribute to the regulation of the normal insulin/glucagon ratio? (1)
☆The normal insulin/glucagon ratio is intricately controlled by nutrients modulated by various factors, including endocrine, paracrine, and neural effects, including the incretin effect.

