-
Coulombs Law says
when the radius is smaller the bonds are stronger
-
Ionic metals
Transfer electrons
Metals and non-metals
-
Molecular Covalent
Share electrons
non-metal elements
-
Metallic
Strong
in a sea of electrons
-
Extended Covalent
Non-metal elements
Lattice like structure of MANY MOLECULES
-
Why is MgBr2 harder than KF?
MgBr2 has a total chare of 2 while KF has a total charge of 1. Compared to Mg K is lower on the periodic table and has an extra shell making it bigger. Based on coulombs law when the radius is smaller the bonds are stronger. MgBr2 have the strongest bond since it has a smaller radius in total.
-
Polar
Electrons being pulled to one side more making that side delta (slightly) negative, and the other delta positive
-
Non-polar
Share electrons
-
Intermolecular forces
Interactions between molecules:
Repulsion, attractions
Ion Dipole
Dipole-Dipole
Hydrogen Bonding
London Dispersion
-
Intermolecular forces are
weak because small or partial charges are interacting over large distances.
Exist between molecules
-
Intramolecular forces are
strong due to large electrostatic interactions over short distances
-
Electron geometry
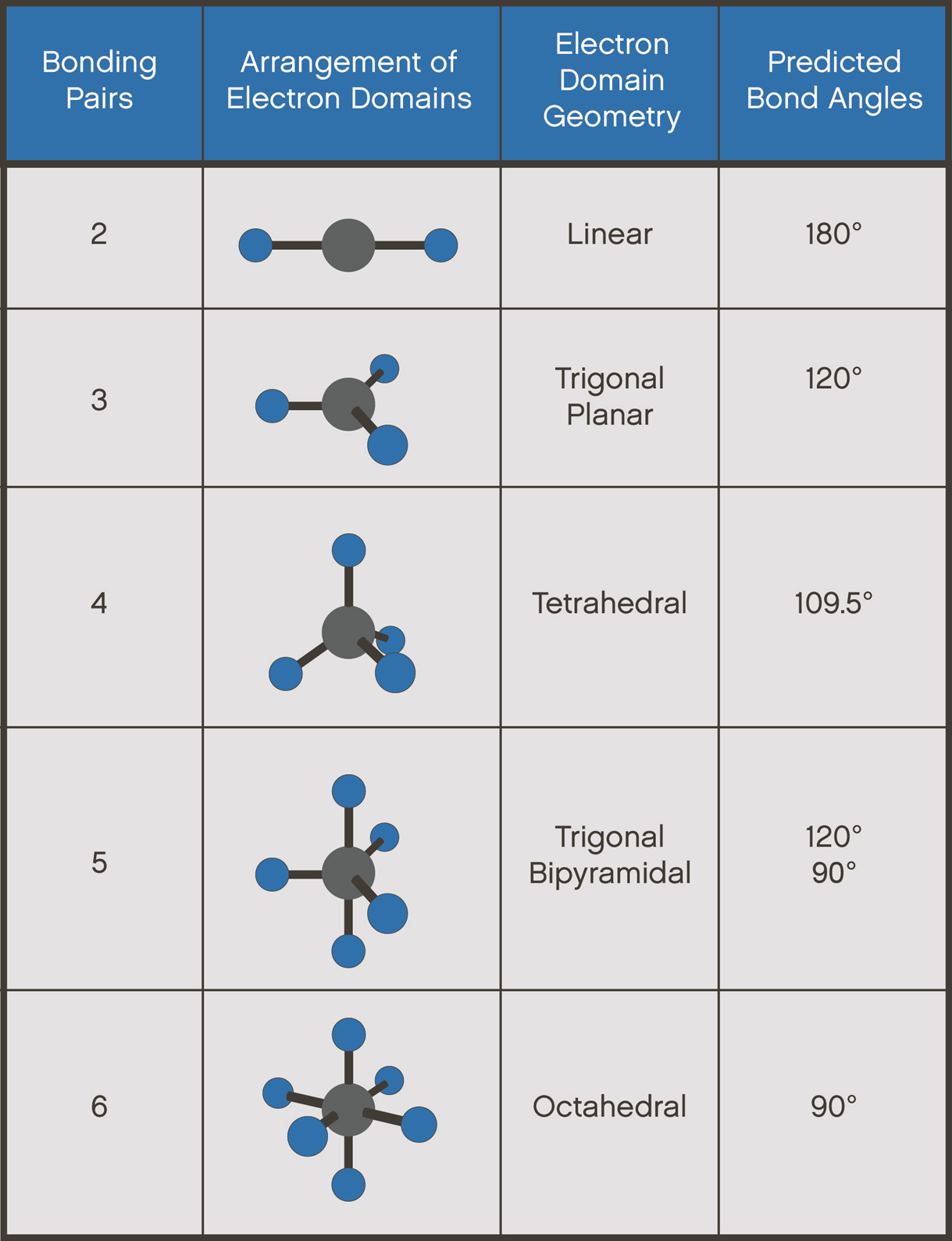
Lone pairs count as a bond
-
Molecular geometry
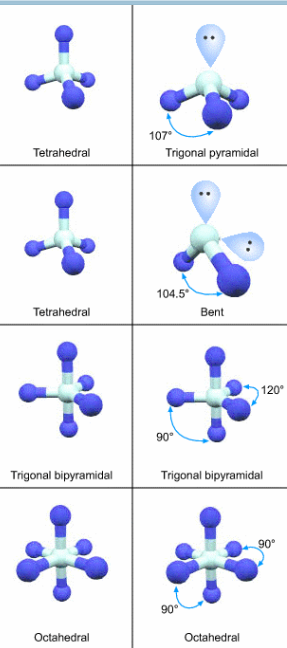
Lone pairs are not counted but they help with form new shape
-
What is the Lewis Dot structure to
Al2O3
O Double bond Al Bond O Bond Al Double bond O (Add lone Pairs to O)
-
Calculate the frequency of light with a wavelength of 756nm
use frequency formula
1. Convert 756 nm to meters
2. Plug into formula
3. Your answer should be 3.96 x 10^15 Hz
-
A photon of light has an energy 1.23 x 10^-19 J. Determine the wavelength of the light wave:
You are given E
Use wavelength = hc/E
You should have gotten
1.65 x 10^-7
-
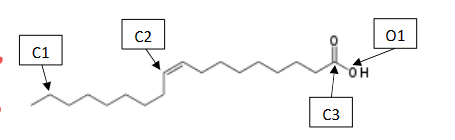
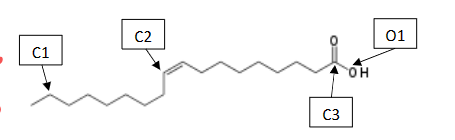
What is the chemical formula of oleic acid?
C18 H34 O2
-
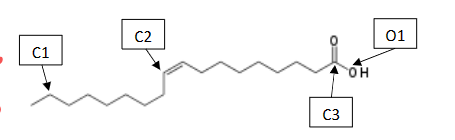
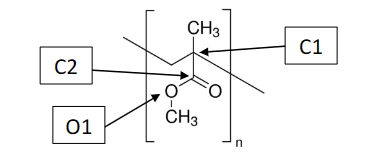
What is the Molecular and Electron Shapes of C1
Molecular: Tetrahedral
Electron: Tetrahedral
-
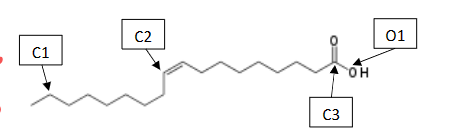
What is the Molecular and Electron Shapes of C2
Molecular : Trigonal Planar
Electron: Trigonal Planar
-
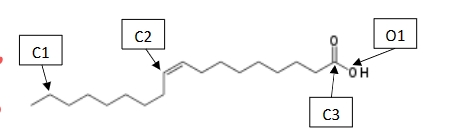
What is the Molecular and Electron Shapes of C3
Molecular : Trigonal Planar
Electron: Trigonal Planar
-
What is the Molecular and Electron Shapes of O1
Molecular: Bent
Electron: Tetrahedral
-
Polar + Polar =
Soluble
-
Polar + Non-polar =
not soluble
-
Non-polar + non-polar =
Soluble
-
What is the Lewis Dot structure for
NO2 ^-
Is it polar?
Shape?
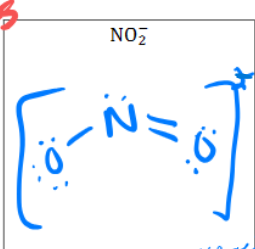
Not polar
Bent
-
What type of bond is happening
London Dispersion?


