-
Energy eqn for the (1s)^2 singlet helium
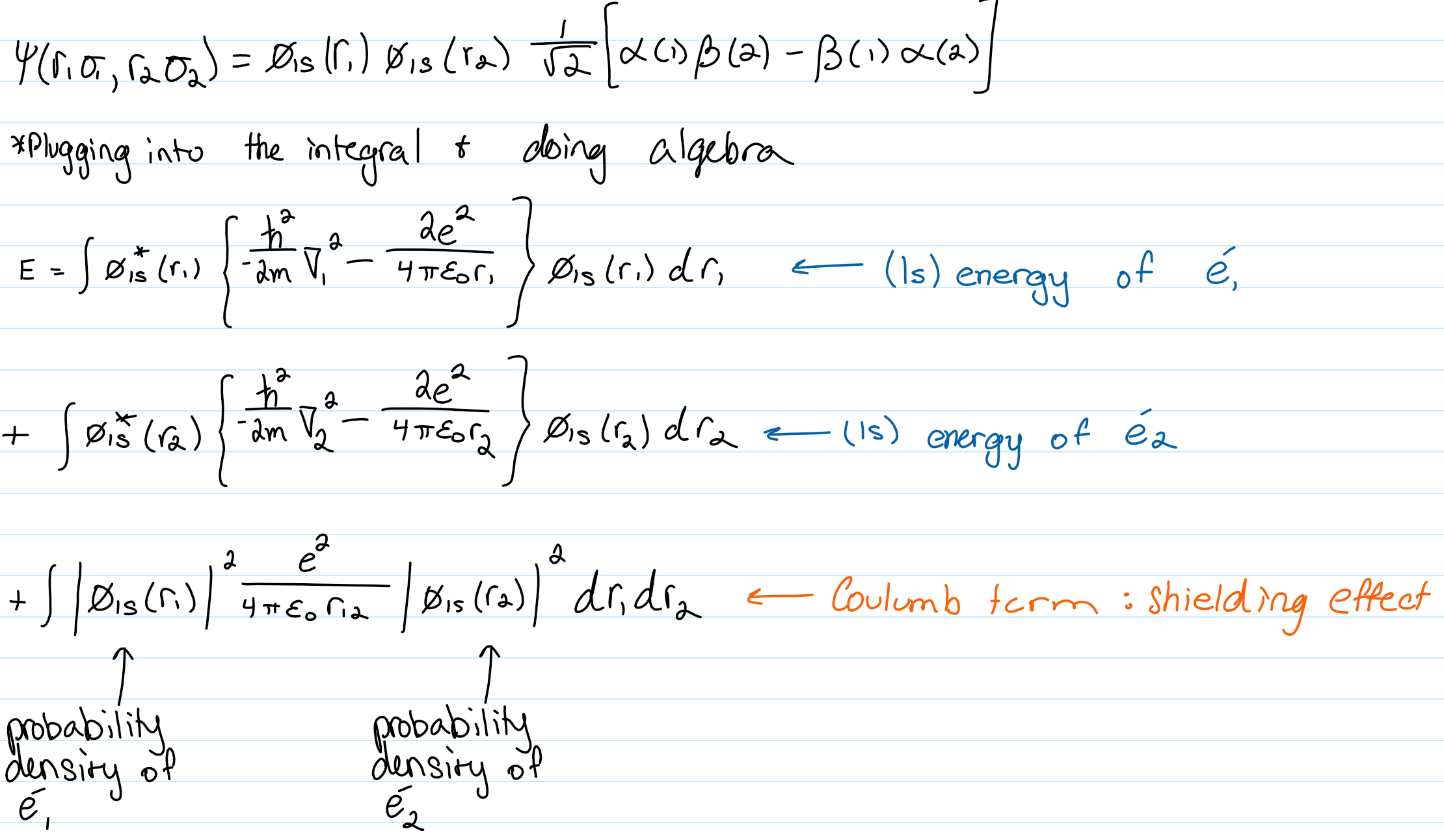
-
Energy eqn for the (1s*alpha)(2s*alpha) triplet helium
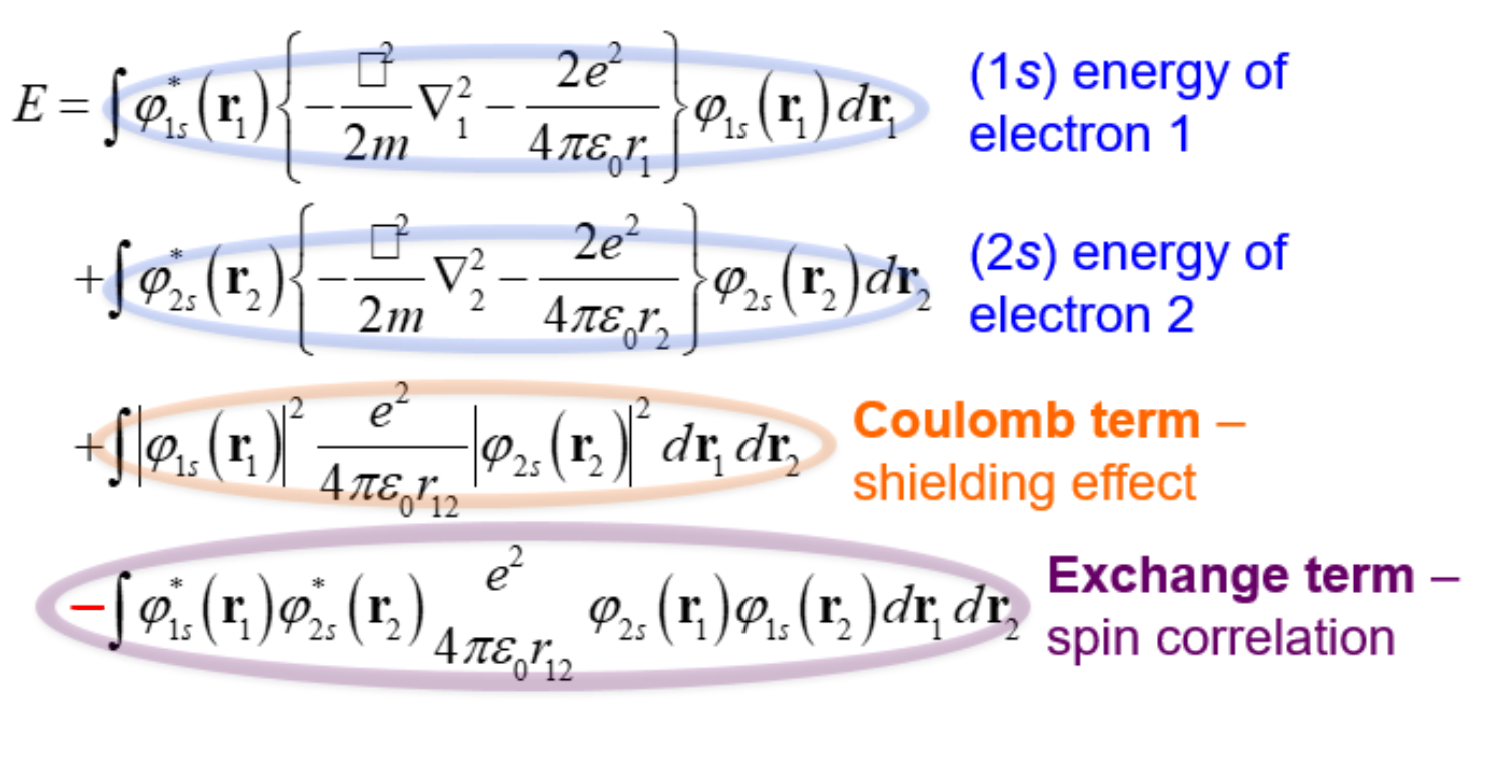
-
Energy eqn for the (1s*alpha)(2s*beta) singlet helium
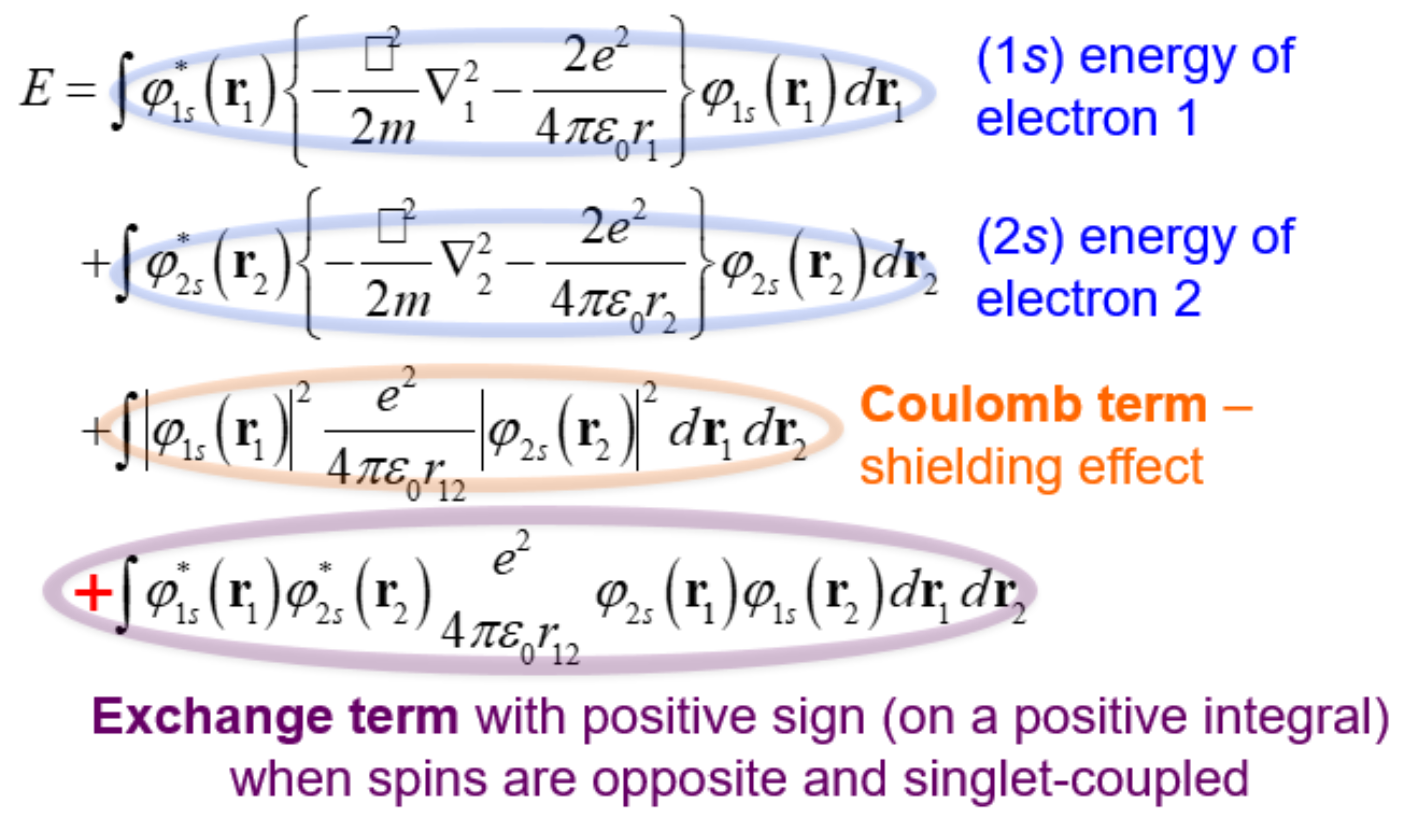
-
What is Hund's Rule
Hund's rule states that the lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell.
-
What are the spin quantum #'s (S, ms)
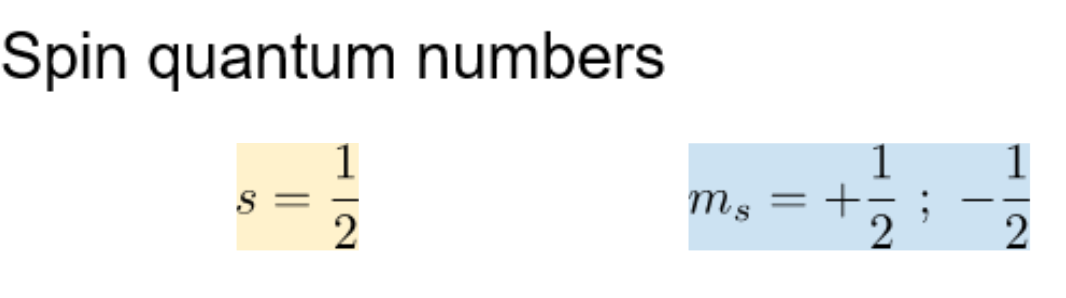
-
What are the spin multiplicities

-
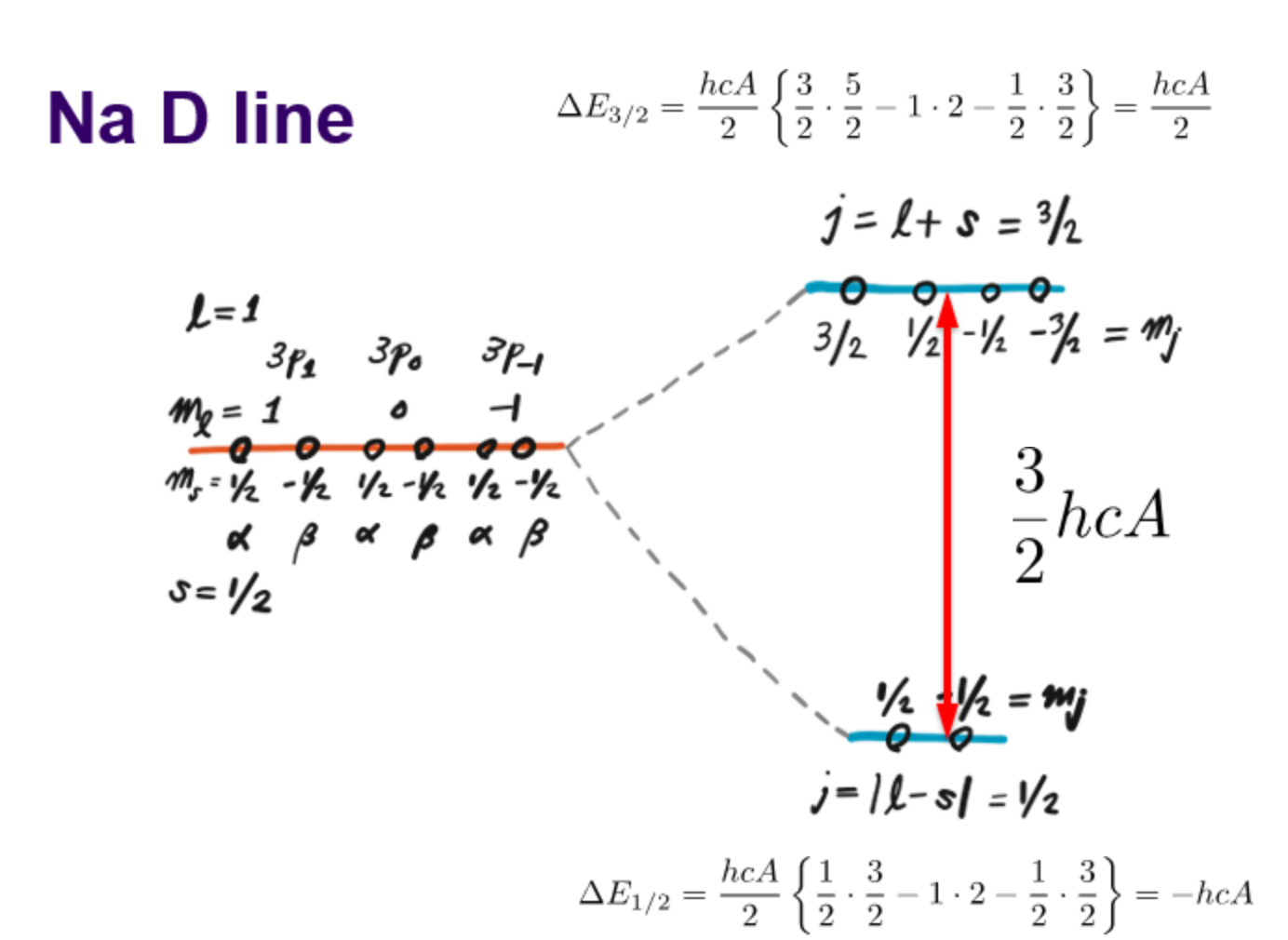
Sodium D line when L = 1
-
Sodium D line when L = 0
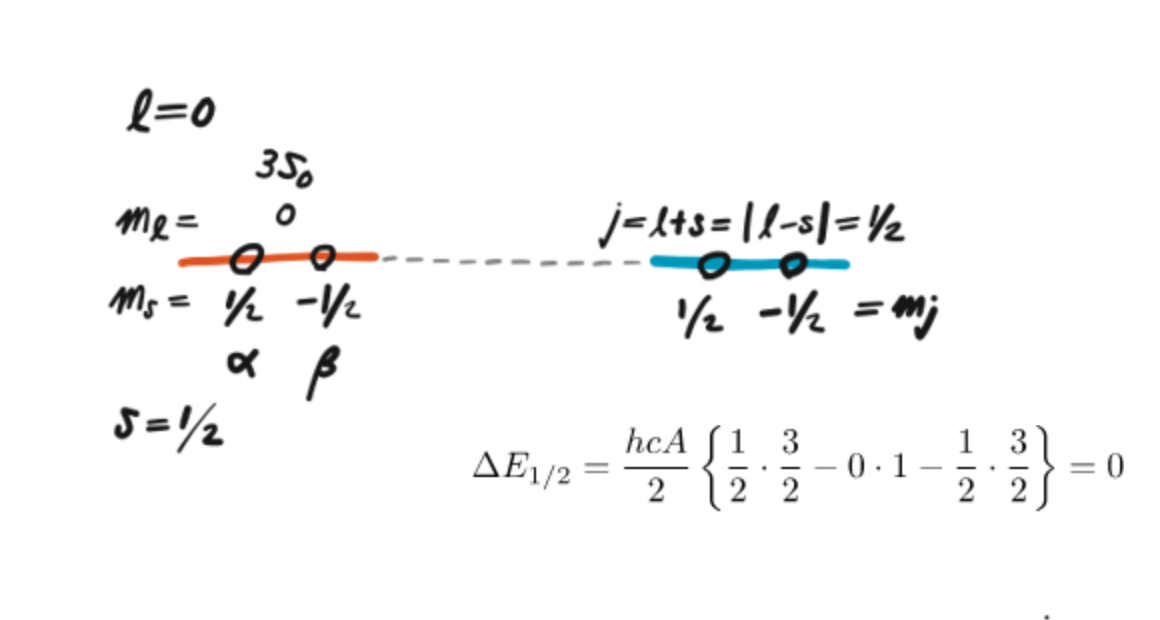
-
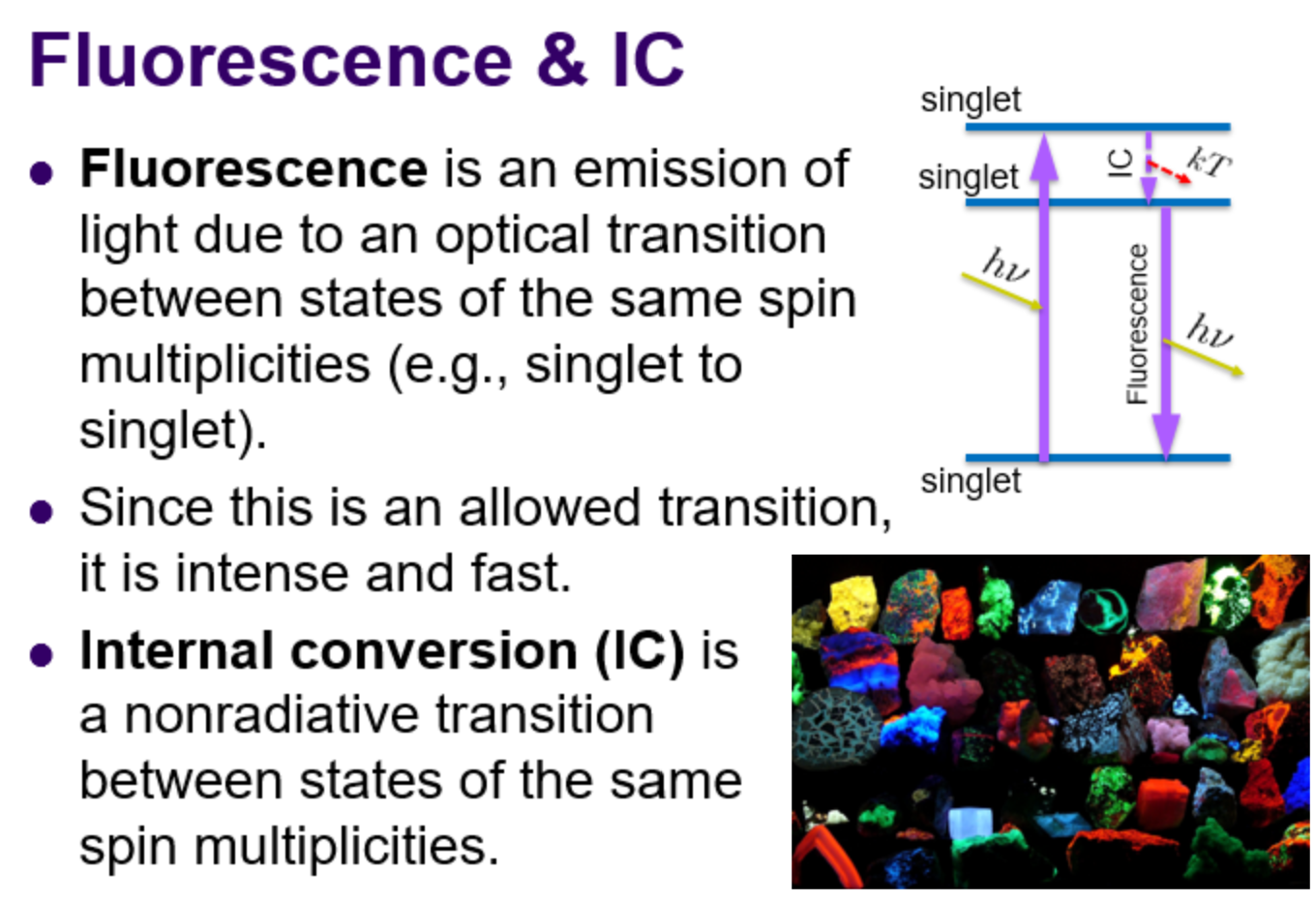
Fluorescence
-
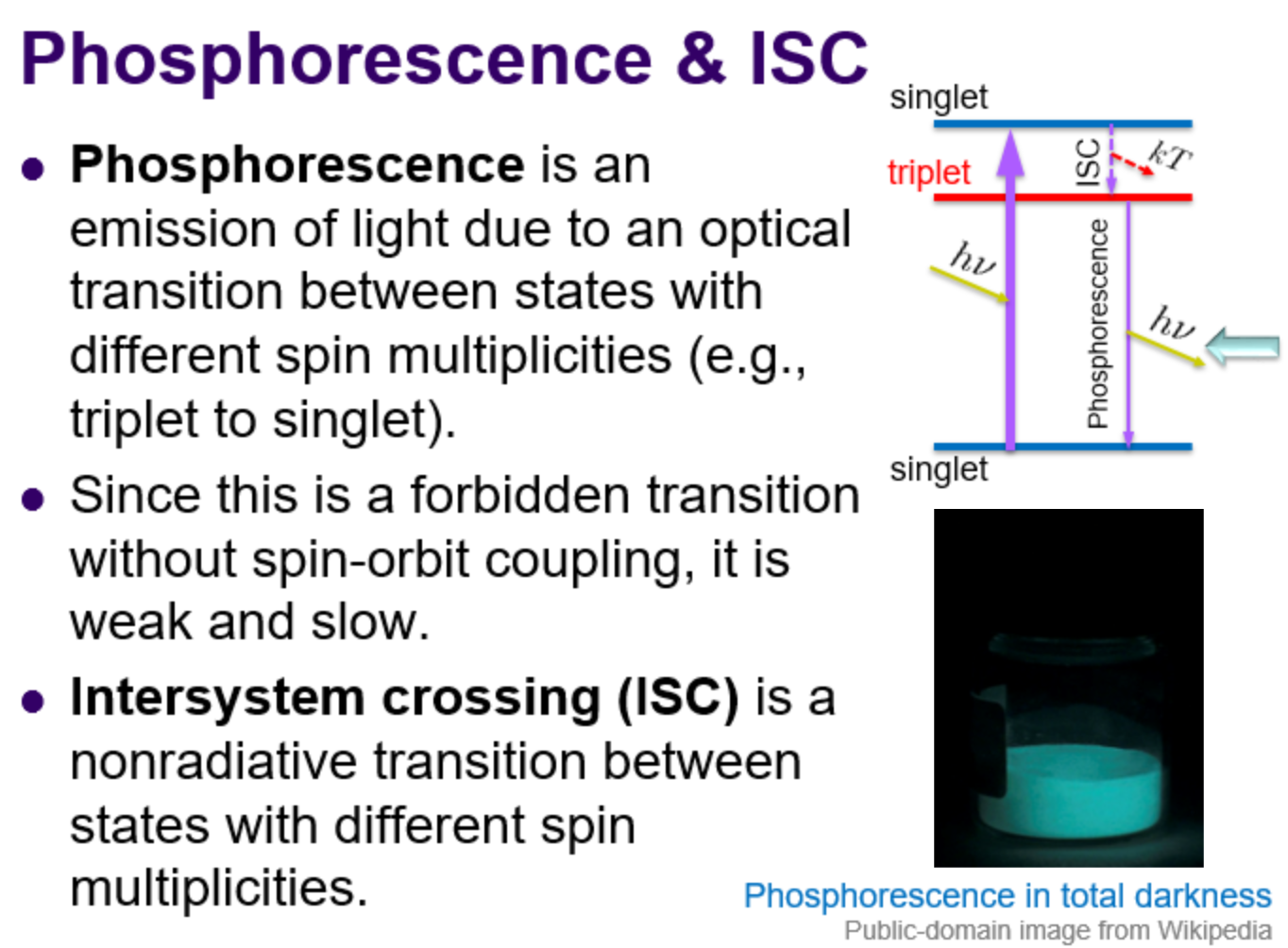
Phosphorescence
-
Angular momenta j,L,S,mL,mS,mJ
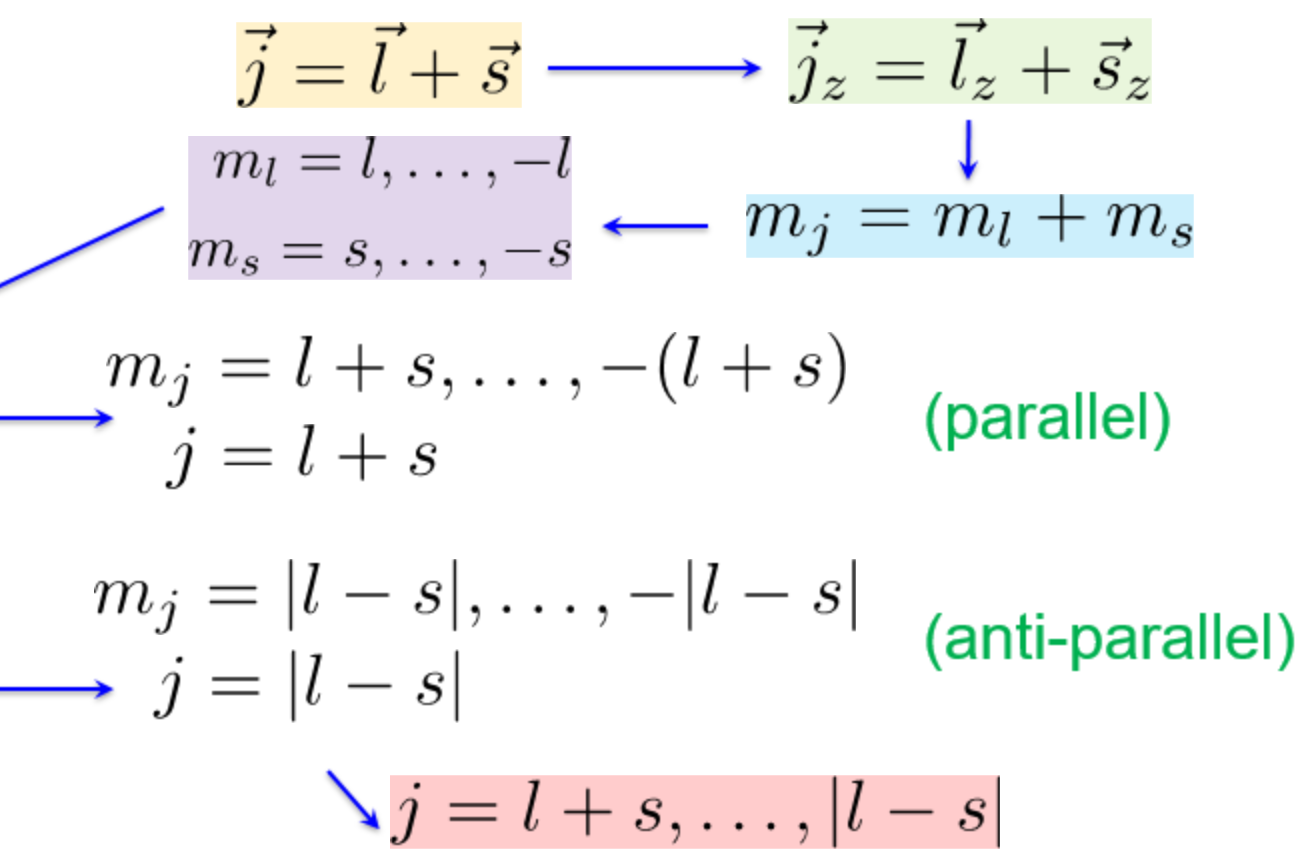
-
Molecular Hamiltonian split into electronic and nucleus portions.
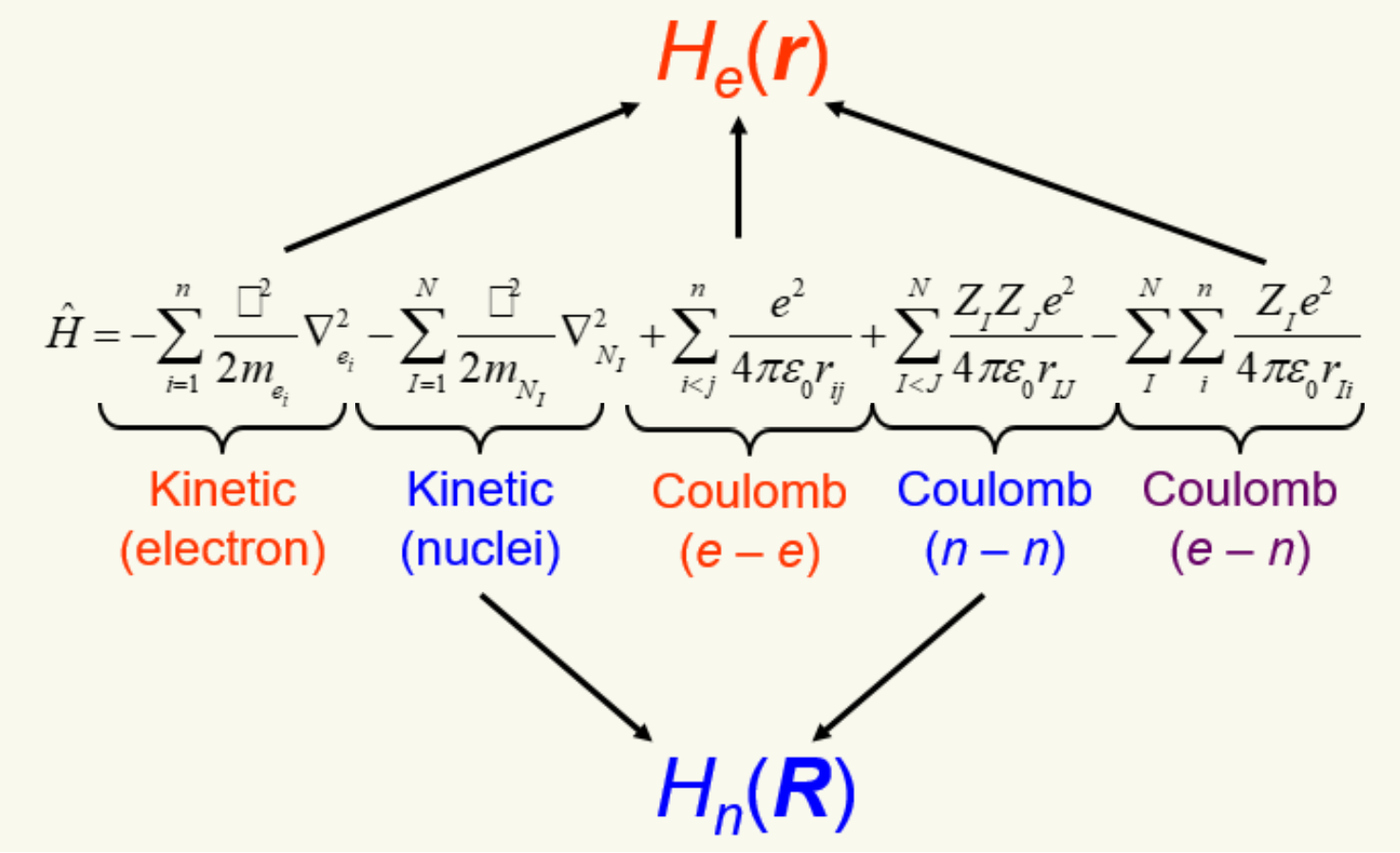
-
Dynamical degrees of freedom

-
Born Oppenheimer approximation
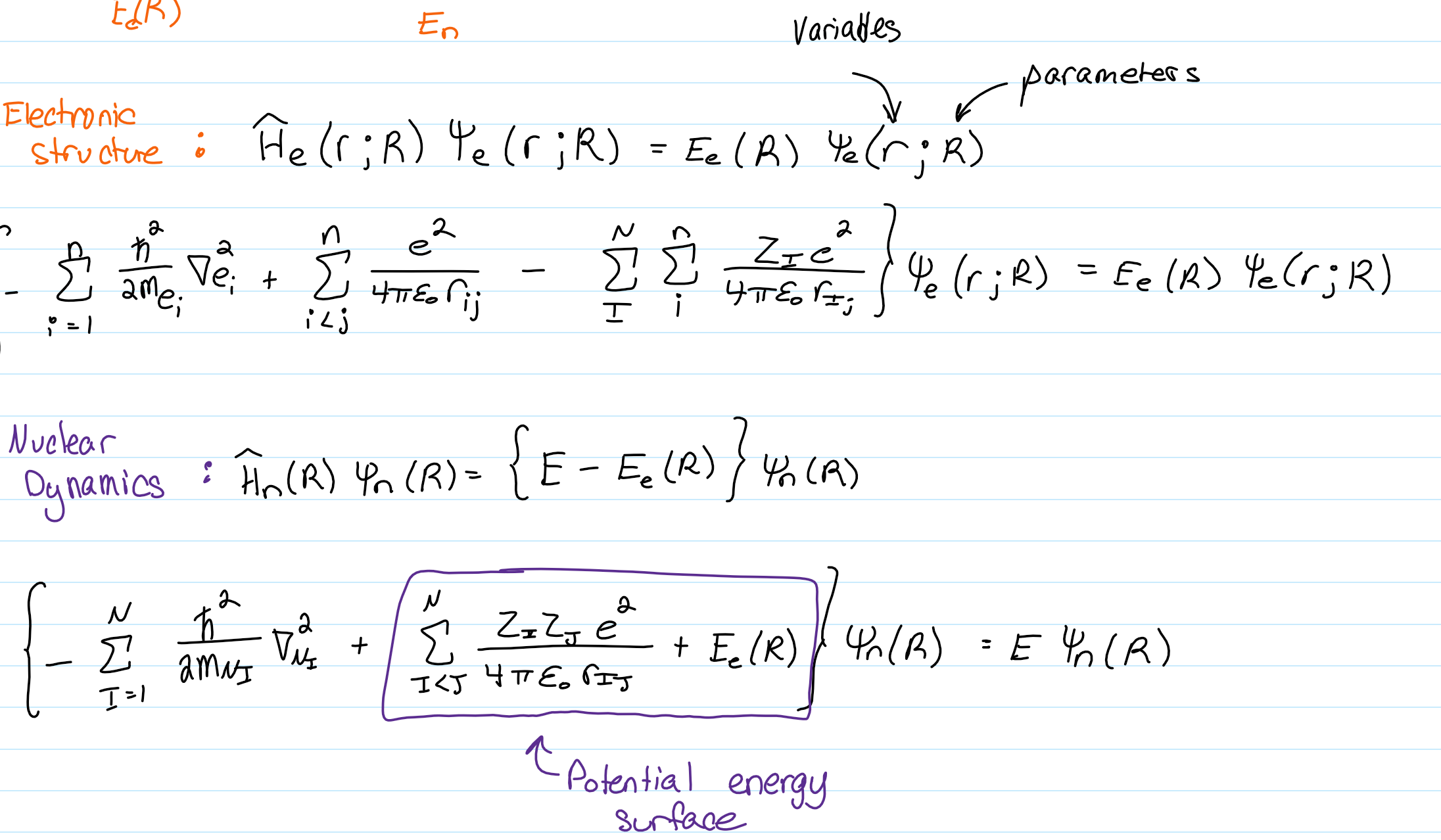
-
What is the VB theory for H2? (singlet and triplet)
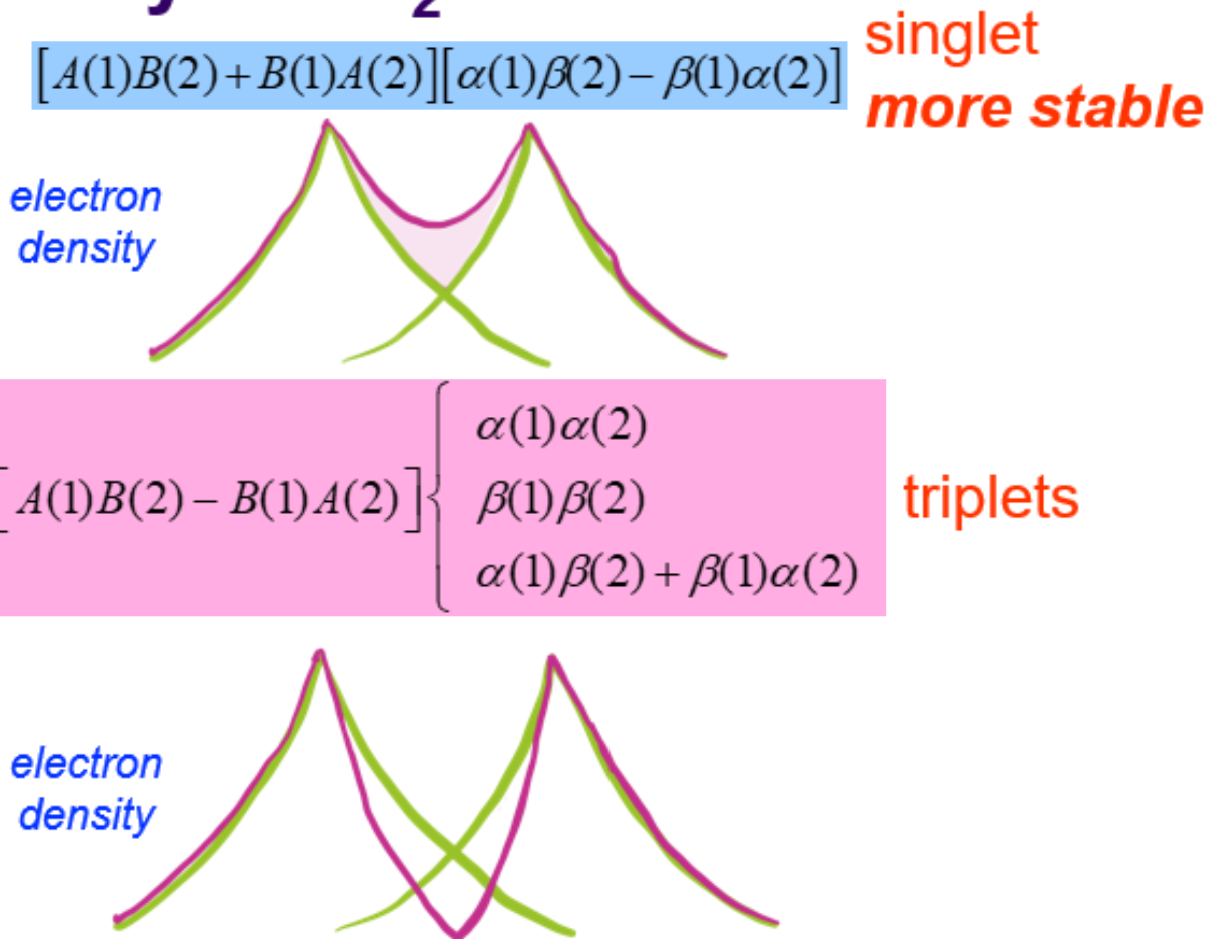
-
What is promotion/hybridization?
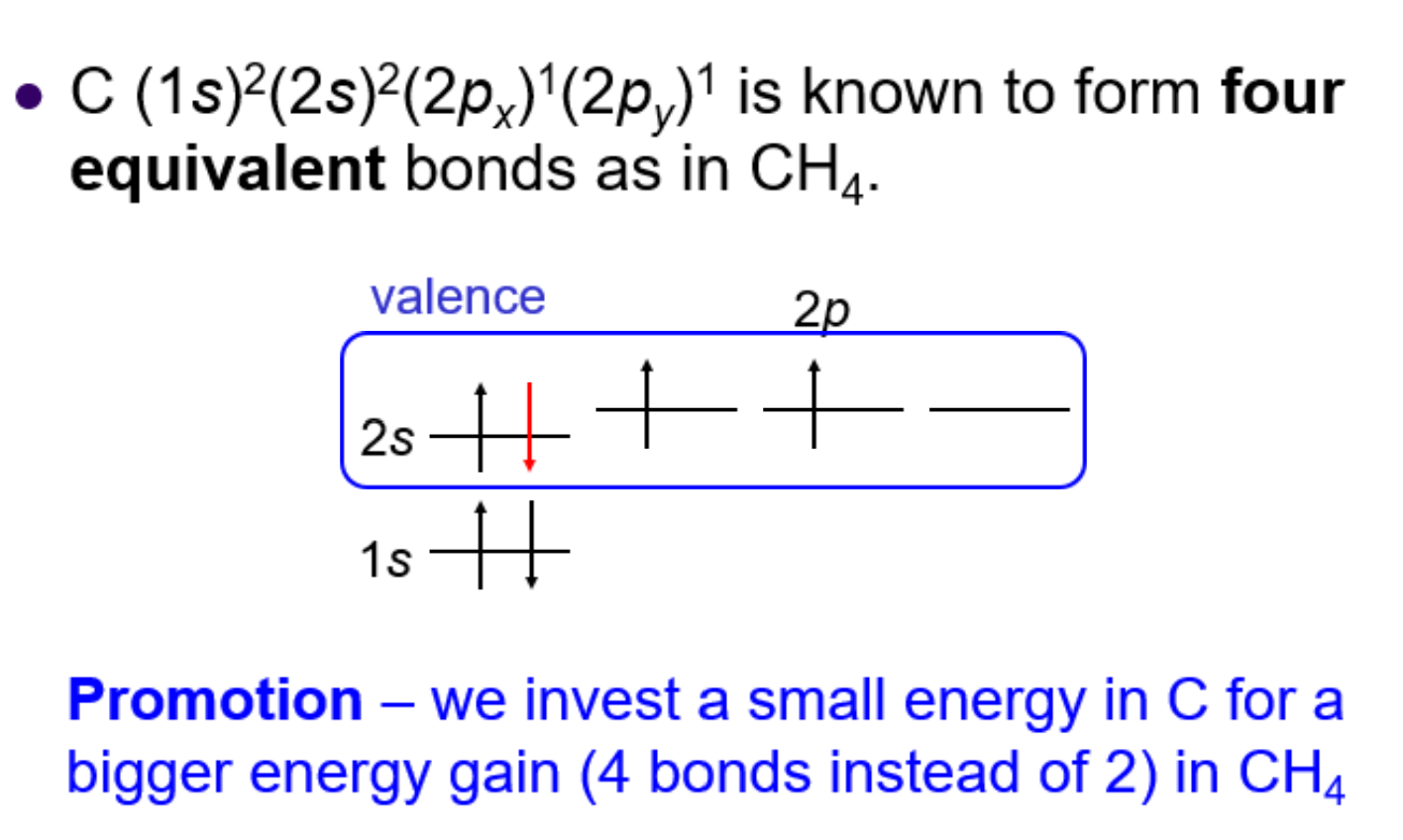
-
What do X and Y represent in MO theory?
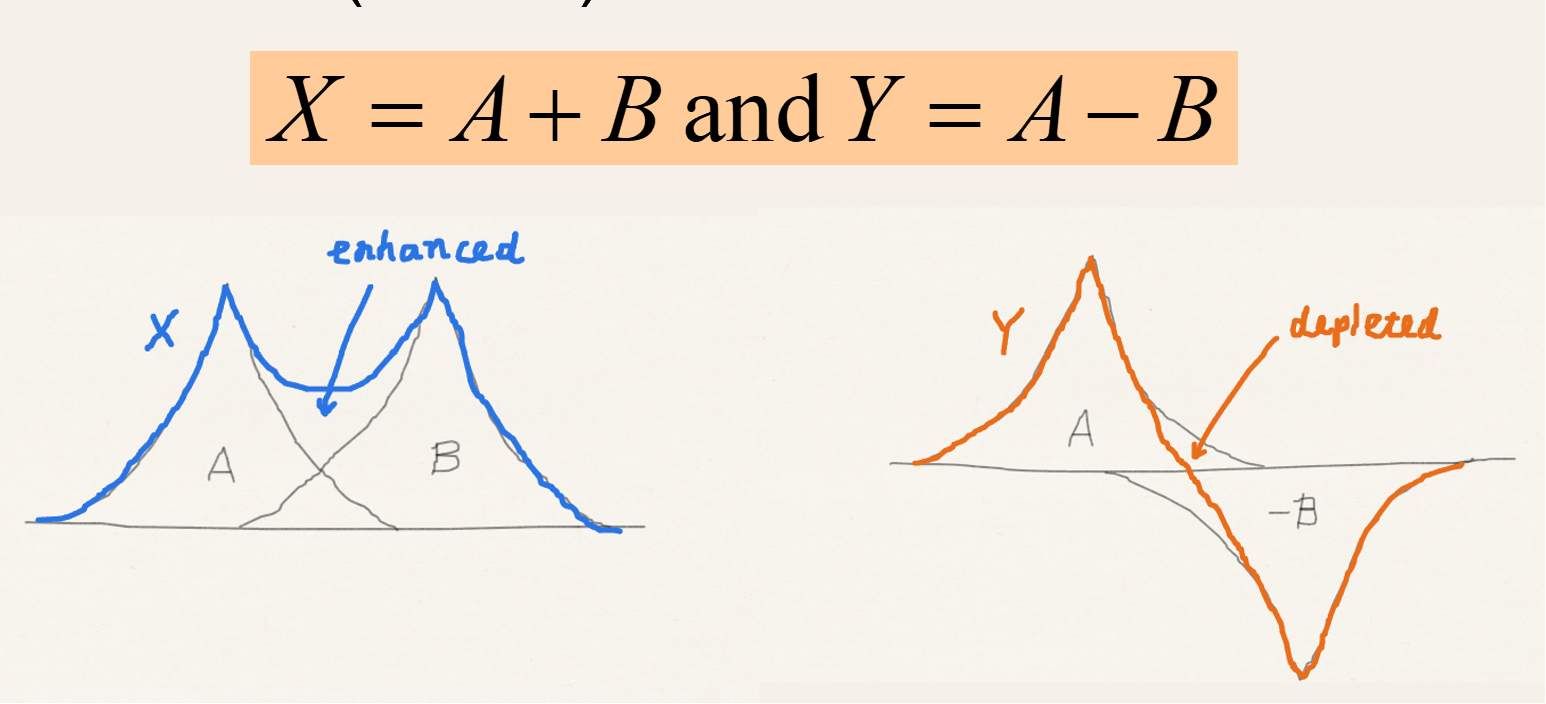
-
What are the singlet and triplet wave functions for H2 in MO theory?
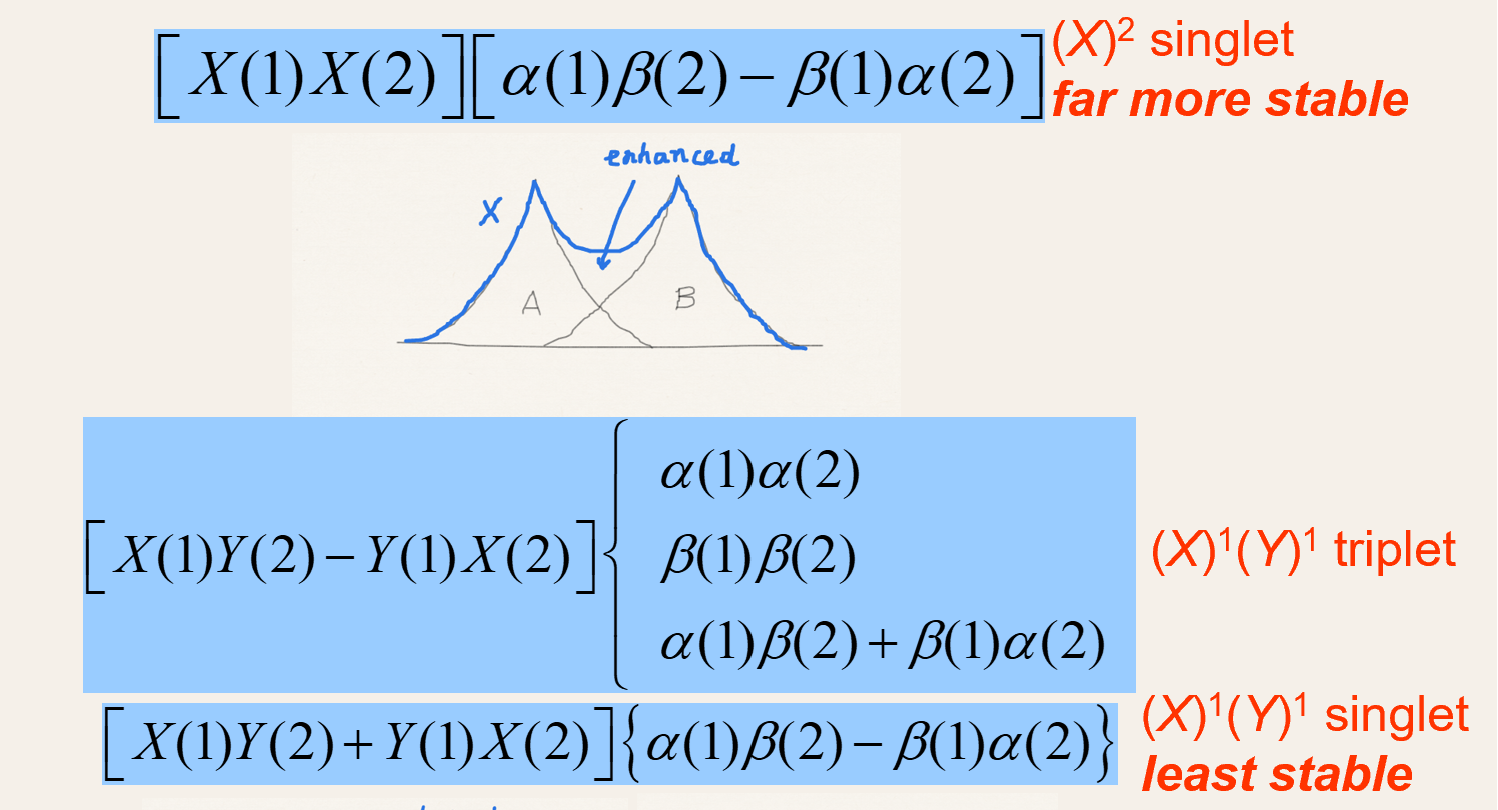
-
What does MO theory account for that VB doesnt?
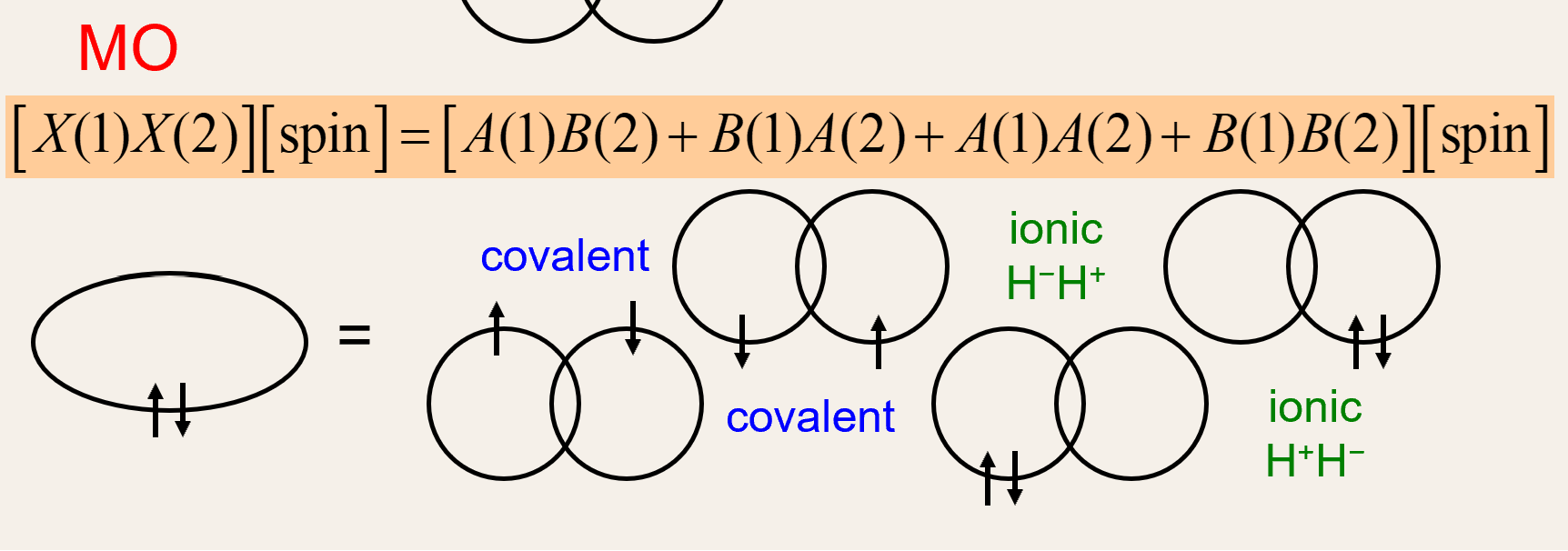
-
Hamiltonian for MO theory
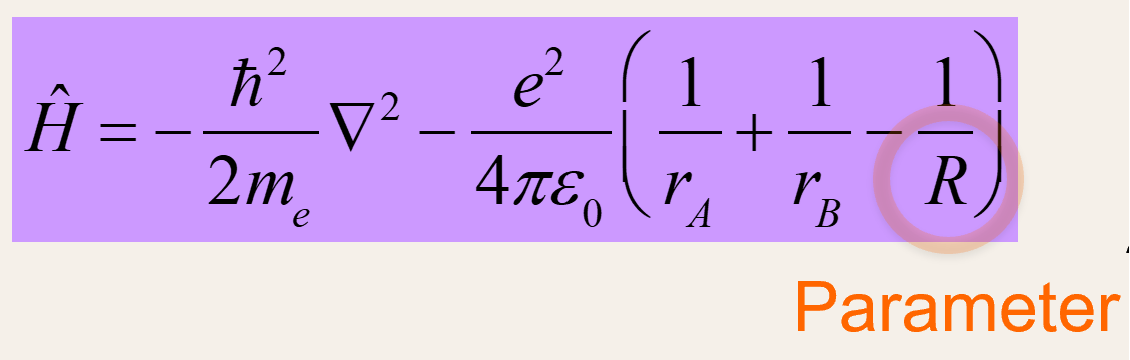
-
Is bonding more bonding that the anti-boding is anti-bonding?
No, anti-bonding is more anti-bonding than bonding is bonding.

