-
accuracy in measurement
how close a set of results is to be true value
-
precision in measurements
how close a set of results are to each other, indicating consistency
-
When is a result consiered both accurate and precise
when values are close to each other and to the true value
-
What does it mean if results are precise but not accurate?
results are consistent but mot close to true value
-
Describe systemic error and give an example?
systemic errors are consistent and directional, often due to flaws in experiment setup
EX: reading volume too high on graduated cylinder every time
-
Random error and ex
random errors are unpredictable and are due to uncontrolled variables
EX: different readings from digital thermometer on same object
-
Absolute error
fixed amount of error in the same unit and decial places as the measurement
-
What is relative error and how is it calculated?
the error as percentage of the measurement
Calc: % Relative Error = (Absolute Error / Measurement) × 100%
-
if a measurement is given as 5.0 cm ± 0.1 cm, what is the absolute error?
± 0.1 cm
-
Calculate the Absolute error of (5.0 ± 0.02) x (0.4000 ± 0.0002)
ΔC = (( ±0.02/ 5.00) + (±0.0002/0.4000)) x (5.00 x 0.3000)
= 0.009
C= AxB —> 5.00x.4000 = 2.00
--> 2.00 ± 0.01
we CANNOT write = 2.00 ± 0.009 round up the value of 0.009 to 0.01 to match precision of 2.00 (2 decial places)
-
How do you calculate the absolute error in result C when multiplying or dividing measurements?
ΔC = (ΔA/A) + (ΔB/ B) x C
C= A x B
-
How do you calculate the Relative error in result C when multiplying or dividing measurements? And what does ΔA&B represent?
ΔC/ C = (ΔA/A + ΔB/B)
ΔA ΔB are the absolute errors in A and B
-
Calculate the relative error of (5.0 ± 0.02) x (0.4000 ± 0.0002)
ΔC/C = (( ±0.02/ 5.00) + (±0.0002/0.4000))
= 4.5 x10-3
= 5x10-3 (rounded)
= ± 0.5% (1 sig fig based on 5)
-
General rules for calculating error propagation when adding or subtracting measurements in relative error
ΔC/ C = (ΔA/C + ΔB/C)
-
General rules for calculating error propagation when adding or subtracting measurements in absolute error
ΔC = (ΔA/C + ΔB/C) x C
C = A-B
-
Calculate the relative error of (5.05± 0.02) - (0.40 ± 0.02)
ΔC/ C = ((±0.02/4.65) + (±0.02/4.65))
= 9x10-3
= ± 0.9% (1 sig fig based on mathematics)
-
Calculate the absolute error of (5.05 ± 0.02) - (0.40 ± 0.02)
ΔC = ((±0.02/4.65) + (±0.02/4.65) x (5.05-0.40)
= 4x10-2
= 4.65 ± 0.04
-
How does systemic error affect %RAD?
It does NOT affects %RAD, as % RAD measures variability, not consistency in one direction
-
How does random error affect %RAD?
it increases %RAD by adding variability to the measurement
-
Using a faculty spectrometer with an unstable light source results in which type of error (random or systemic)?
Random error due to unpredictable fluctuations in the light source affecting each measurement differently
-
Blowing out liquid from a TD pipet during calibration results in which type of error?
Systemic error, as it cosistently affects the volume delivered in one direction (too much liquid)
-
What is the minimum (nonzero) absolute error for a 0.50M KCL solution?
±0.01
-
A graduated cylinder has a 2% relative error. A student measured out a 15mL HCl solution using a graduated cylinder. Determine the number of significantfigures that need to be reported in the volume measurement in order to satisfy the 2% relative error.
absolute error found to be 0.3mL and absolute error is the fixed amount of error with the same amount of units and decimal places, the measurement should reported as 15.0mL with 3 sig figs to satisfy the 2% relative error requirement.
-
Is there anything incorrect with the following measurement? 2.50g ± 0.08%
the absolute error cannot be in percentage form. if calculating for the relative error, must be done seperately.
-
How many sig figs should be reported for a 5-mL volumetric pipet?
5.00 mL (3 sig figs)
-
How many significant figures should be reported for a 10-mL volumetric pipet?
10.00 (4 sigs figs)
-
How many significant figures should be reported for a 100-mL volumetric flask?
100.00mL (5 sig figs)
-
What decimal place should you report measurements in mL for volumetric glassware. provide example
to the hundredth decimal place in mL
EX: the volume of a 100-mL volumetric flask is equivalent to 100.00mL OR 0.10000L (both values have the same number of significant figures!)
-
does the number of decimal places change when converting from mL to L
yes, deciamal places change due to unit conversion, but sig figs remains the same
-
What is the typical precision range for Beer’s Law in spectrophotometric analysis?
Semi-quantitative provides approximate, rather than exact results, not perfectly precise, with a range of ±5 to 10%
-
Advantages of using spectrophotometer in analysis?
rapid, allows for quick measurements
-
How does spectrophotometry support quality control as an advantage?
it’s easy to establigh quality control measurements, making results mroe reliable?
-
How does Beer’s Law benefit real time monitoring as an advantage?
can be used for on-line monitoring to continuously track changes
-
Key challenege when using spectophotometry with Beer’s Law?
the absorption profile of the chemical of interest must be distinct from other solution constituents to avoid interference
-
What equation relates the intensity of incident light (I0) and transmitted light (I) for dilute solution?
I = I0 X 10-ϵCL
-
What does I0 represent in the Beer-Lambert Law?
I0 is the intensity of the incident light beam
-
What does I represent in the Beer-Lamert Law?
I is the intensity of the transmitted light beam
-
What is ϵ in the Beer-Lambert Law & units?
ϵ is the molar absorptivity constant (units: L/(mol·cm) or M−1cm−1).
-
What does ϵ depend on?
ϵ depends on the identity of the chemical species and the wavelength of light selected.
-
What does C represent in the Beer-Lambert Law?
C is the concentration of the chemical species in the solution (in mol/L).
-
What does L represent in the Beer-Lambert Law?
L is the depth of the solution that the light beam traverses (in cm).
-
What is Transmittance (T), and how is it calculated?
Transmittance (T) is the ratio of transmitted light to incident light, calculated as T=I/I0=10−ϵCL, where I0 is the intensity of incident light and I is the intensity of transmitted
-
What is the formula for Absorbance (A) in terms of Transmittance (T)?
Absorbance (A) is calculated as A=−log(T)
-
What is the relationship between Absorbance (A), molar absorptivity (ϵ), concentration (C), and path length (L) in Beer’s Law?
A=ϵCL, which is Beer’s Law.
-
When is ϵ considered a constant?
Wavelength (λ) is fixed during the analysis.
The sample has a low concentration.
-
What is meant by "low concentration" in Beer’s Law?
Low concentration is ambiguous, but it generally refers to concentrations where Beer’s Law holds without deviations.
-
If ϵ is constant, what is the relationship between Absorbance (A) and Concentration (C)?
Absorbance (A) is directly proportional to Concentration (C).
-
What is the acceptable range for Absorbance (A) in Beer’s Law?
Absorbance (A) should fall between 0.1 and 1.0 for accurate results.
-
How many sig figs are supposed to be used when adding
ex: 39.64 + 1.3
The limiting factor with the number of decimal places
ex answer: 40.9 —> 1 sig fig
-
How many sig figs are supposed to be used when subtracting
ex: 195.4 – 193
the limiting factor
ex answer: 193 —> no sig figs
-
How do you add exponents & how many sig figs?
ex: 5.85×102 + 1.21×104
can’t add or subtract if the values have different exponents in since it affects decimal place, change exponents so they match
ex: 5.85×102 + 121×102 = 1.27 x104
-
How do you calculate sig figs when multiplying or dividing?
ex: (3.84×21.69) ÷ (2.9×1.63)
find limiting factor and use sig figs not decimal place
ex answer: limiting factor 2.9 w/ 2 sig figs, answer —> 18
-
If the beaker containing a sample of alcohol weighs 49.8767 g and the empty beaker weighs 49.2140 g, what isthe net weight of the alcohol
49.8767 g - 49.2140 g =0.663 g 0.6627
2 SF
-
How to calculate percentage RAD and what does RAD stand for?
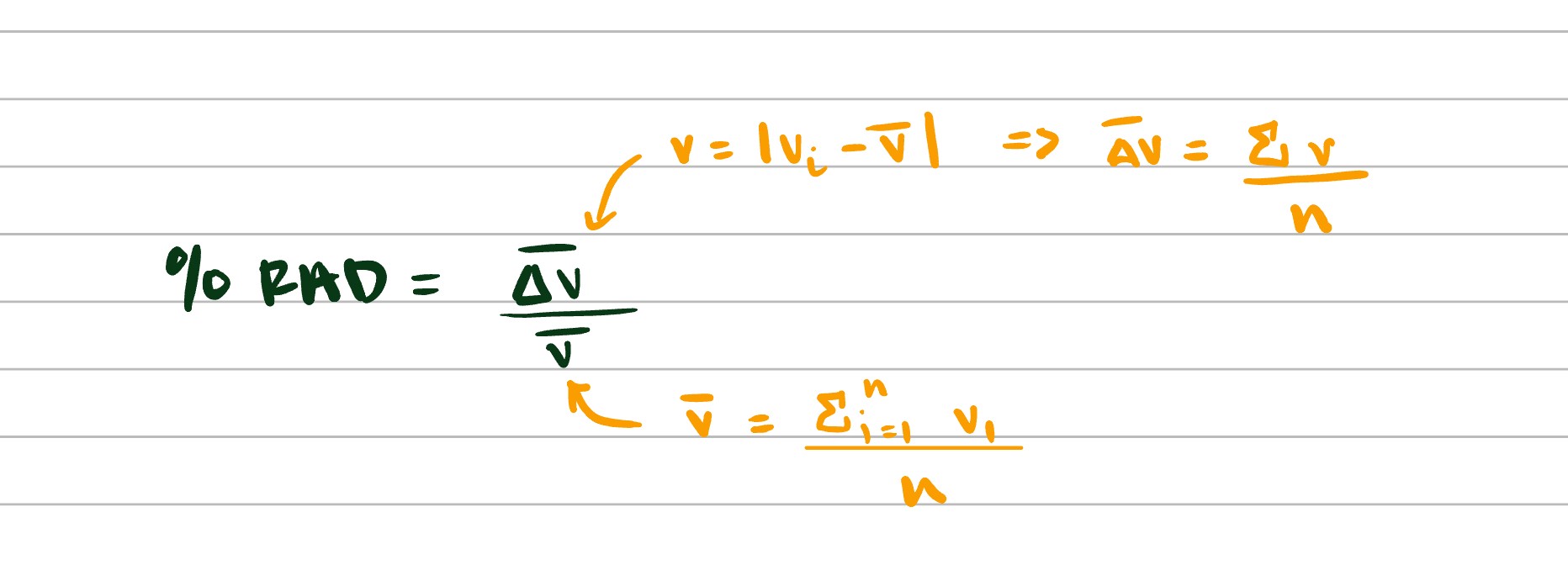
Relative Average Deviation
-
When is it necessary to consider rejecting an observation from a small data set?
When one result deviates significantly from others, potentially causing large error in the average, especially if repeating the experiment isn’t possible
-
What basis justifies rejecting a result?
identifiable systemic error in the measurement or if the statistical deviation indicates low probabilty of the results fitting with the rest of the data
-
What is the deviation ratio test?
statistical method determines if a single deviant result in a small data set should be rejected based on how much it deviates from the others
-
What is the formula for the Deviation ration (Rq) test?
Rq=∣xq−xn∣ / ∣xq−xf∣
xq = questionable result
xn = result nearest to xq
xf = result farthest from xq
-
How does Rq compared to determine if a result should be rejected?
Rq is compared to a critical ration (found in chart IV 7) specific to the number or measurements (n<7), if Rq greater, result likely error
-
Draw graphical representation of Beer’s Law graph with constant wavelength
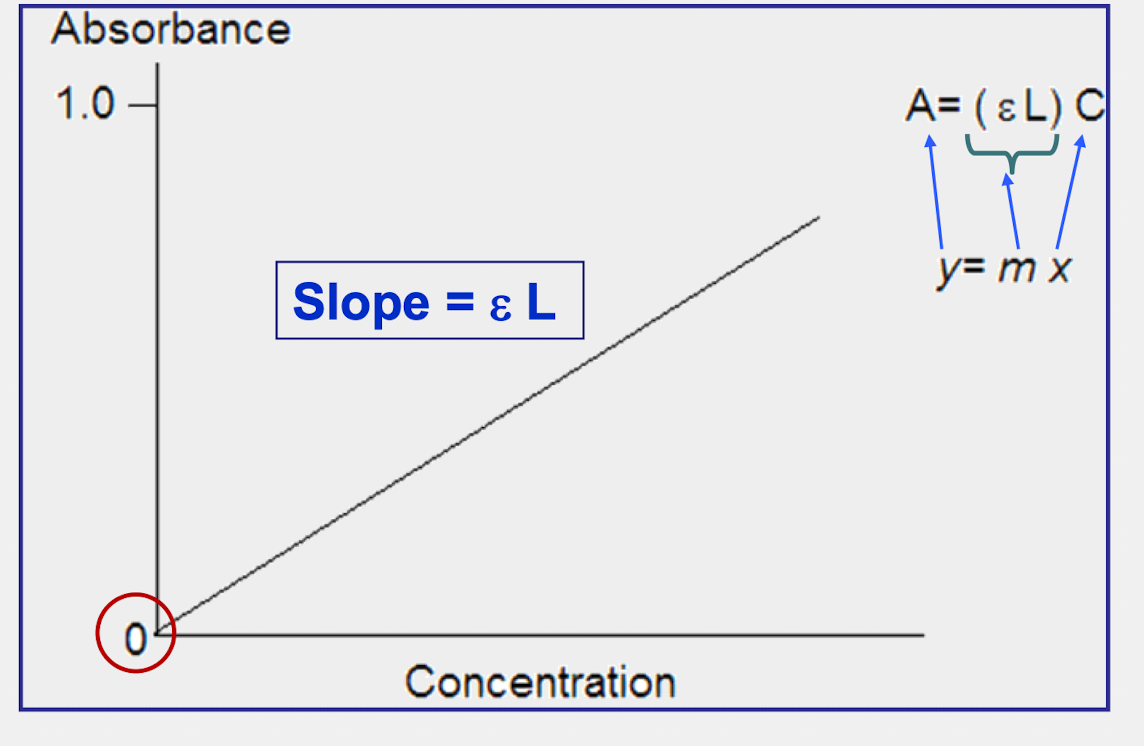
graph must start at 0,0 bc of the blank reading
-
How to calculate the slope using A = ϵLC
slope = ϵL
or = yf-yi / xf-xi
-
What if Beer’s Law experiment graph is not linear?
if curved or non-linear means Beer’s Law failed and concentration may be too high
-
Beer’s Law doesn’t work if the solution concentration is too high. How do we solve this lab issue?
at high concentrations, solution absorbs to much light that absorbance and concentration (beer’s Law) no longer accurate. Dilute the solution until absorbance falls between 0.1 to 1.0
-
if Slope of the line on the Beer’s law calibration graph = ϵL, how do you calculate the molar absorption constant?
ϵ = slope/ L
-
What does it mean when a standard solution "absorbs" a certain color of light?
it "takes in" that color and doesn't reflect it back. Instead, we see the opposite (complementary) color.
-
What is a complementary color?
the opposite color to the one absorbed. For example, if yellow is absorbed, we see purple.
-
Purpose of crystalization (recrystallization)?
to purify an impure product by forming crystals of the pure compound
-
Basic principle of crystallization?
soluability increases with temp, so compound dissolves better in hot solvent and forms crystals as it cools
-
What challenges exist in crystalization
impurities can have simililar soluability to the desired product, makaing them hard to seperate
-
What is the basic procedure for crystalization with a single solvent ?
1. dissole impure product in minimum hot solvent to make saturated solution
2. filter to remove insoluable impurities
2. Cool solution to form crystals
4. Filter crystals to get purified product
-
Why use the minimum amount of solvent in crystalization?
helps make the solution more saturated so impurities stay dissolved, and more pure crystal forms
-
4 requirements for a good crystallization solvent?
Dissolve a lot of the product at high temperature
Not dissolve the product at low temperature
Dissolve impurities at all temperatures
Not react with the product
-
Why might a mixed solvent system work better than a single solvent system?
Two solvents can balance solubility better: one with high solubility and one with low solubility for the product, making crystallization more effective.
-
What is required for a mixed solvent system to work?
The two solvents must:
Be miscible (mix well together)
High solubility solvent dissolves the product easily
Low solubility solvent dissolves the product less, helping crystals form
-
What are the steps for crystallization in a mixed solvent system?
Dissolve the product in the high-solubility solvent at high temperature.
Add low-solubility solvent slowly until the solution turns cloudy (crystals start forming).
Reheat to dissolve any crystals if needed; add a few drops of the high-solubility solvent if necessary.
-
Why use NaOH to analyze aspirin?
NaOH reacts with aspirin in a 1:1 ratio to neutralize it. An acid-base indicator helps track this reaction by changing color at the end point.
-
What is an end point in titration?
It’s the point where the indicator changes color to show that the reaction is complete. It should be very close to the equivalence point.
-
What is the equivalence point?
point where moles of acidic protons (H+) equal moles of hydroxide (OH-). In the equation, this is expressed as MₐVₐ = MᵦVᵦ (where M = molarity, V = volume).
-
Why is indicator choice important in titration?
The indicator’s color change (end point) should occur close to the equivalence point pH to accurately signal the reaction’s completion.
-
What setup is used for aspirin titration?
Use a buret with NaOH of known concentration to measure volumes accurately, recording to 2 decimal places. The end point tells us moles of NaOH = moles of aspirin.
-
What are intermolecular forces?
the attractive forces between molecules that hold crystalline structures together. They require energy (heat) to break.
-
What is the melting point?
the temperature at which a solid becomes a liquid. It can help determine the identity and purity of a substance.
-
How does melting point indicate identity?
Melting point alone doesn’t confirm identity because many organic compounds may have similar melting points.
-
How does melting point indicate purity?
A sharp melting point range indicates a pure compound, while a wider range suggests impurities. Pure substances typically melt within a range of ≤ 2°C.
-
What is the melting point range for a pure substance?
The melting point range for a pure substance is usually very narrow, and in lab settings, it’s recorded as two temperatures:
Ti: First sign of melting
Tf: Complete meltingThe range is calculated as ΔT=Tf−TiΔT=Tf−Ti.
-
How do intermolecular forces affect melting and boiling points?
The strength of intermolecular forces influences both melting points and boiling points; stronger forces generally result in higher melting and boiling points.
-
what is w/v%?
Weight/volume percent (w/v%) is defined as the weight (in grams) of a substance per 100 mL of solution.
-
Example of w/v%: What does a 5.0% (w/v) NaCl solution mean?
It means there are 5.0 grams of NaCl dissolved in 100 mL of solution.
-
How can you convert grams in a different volume to w/v%?
For example, a solution with 5.0 g NaCl in 275 mL
concentration = 5g / 275 mL =0.0182 g/mL 0.018 g/mL
this is equivalent to 1.8g in 100mL, or 1.8% (w/v%)
-
What is ppm?
Parts per million (ppm) is defined as milligrams of solute per liter of solution (1 mg = 10−3 g).
-
What is ppb?
Parts per billion (ppb) is defined as micrograms of solute per liter of solution (1 μg = 10−6 g).
-
How do you convert molarity (M) to w/v% (g/100mL) ?
1. convert molarity (mol/L) to g/L using molar mass
2. divide g/L result by 1000 to get g/mL
3. multiply g/mL by 100 to obtain w/v% (g/100mL)
** no need to worry about sig figs
-
How do you convert molarity (M) to ppm (mg/L)
convert molarity (mol/L) to g/L using molar mass
ultiple g/L by 1000 to get ppm (mg/L)
-
theoretical yield formula
theoretical yield = mols of limiting reactant x molar mass of product
(actual yield/ theoretical) 100%
-
Actual yield formula
actual yiel = theoretical x % yield
-
Percentage yield
% yield = (actual yield/ theoretical ) 100
-
formula to calculate the volume of solvent required based on solubility
V = m/s
V is the volume of solvent required (in mL),
mm is the mass of the solute to be dissolved (in g),
S is the solubility of the solute in the solvent at a given temperature (in g/mL).
-
London Forces
weak dispersion forces that occur due to temporary dipoles in non-polar molecules. they increase with size of the molecule
-
Dipole forces
occur between molecules that have permanent dipoles (ex: polar molecules).
EX: bond in carbonyl (C=O) where they are partial charges ((δ+ and δ−).
-
Hydrogen bonding
strong type of of dipole-diple interactoin occurs when H is bonded to highly electronegative atoms O,N,F