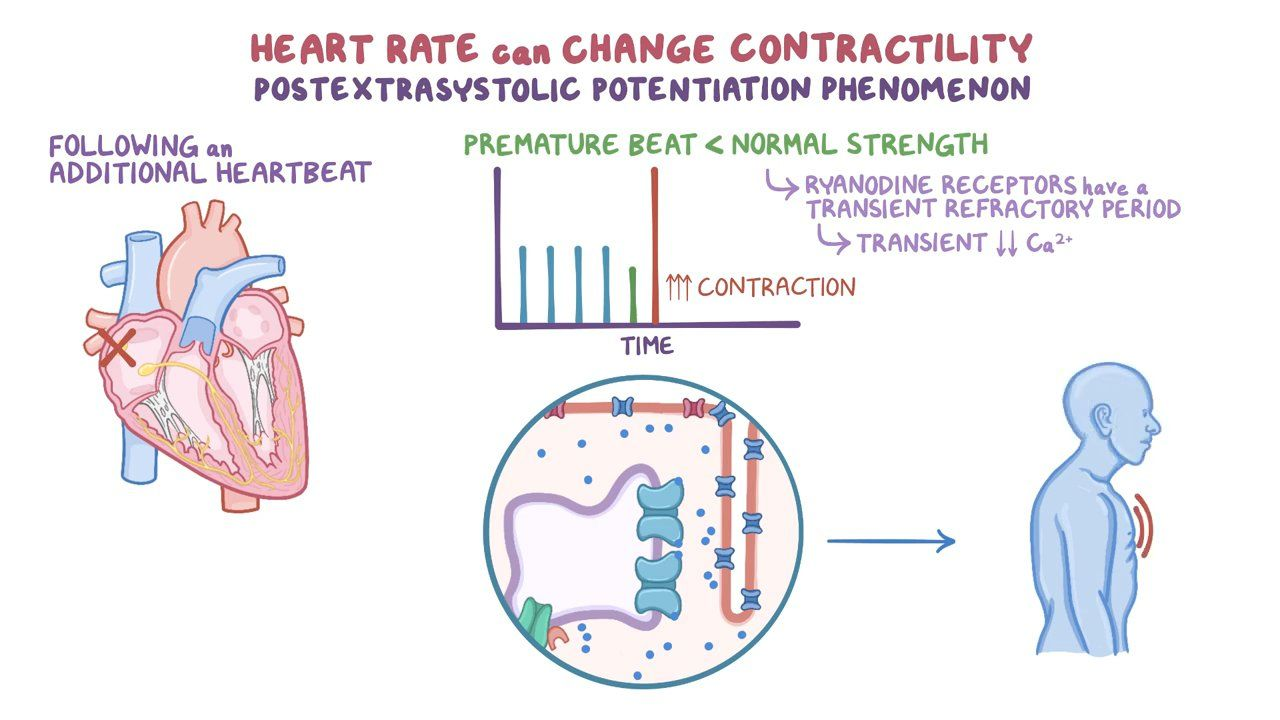
Cardiac Contraction (Physiology)
Session Summary The underlying factor controlling contractility in the heart is the concentration of calcium ions inside the cell. Internal calcium levels rise during contraction. The nature of these rises relate to the type and force of contraction. This lecture deals with the mechanisms involved in cardiac contraction and briefly describes some pharmaceutical approaches to modulating cardiac contractility that may be clinically useful. Learning outcomes At the end of this session you will be able to: Describe mechanisms which increase and decrease intracellular Ca2+. Understand how changes in intracellular Ca2+ concentration relate to contraction. Outline mechanisms by which drugs can increase cardiac contractility.
-
What is the length and diameter of cardiomyocytes (in micrometeres)
60–140 μm in length and 17–25 μm diameter
-
Describe the structure of a myocyte (2)
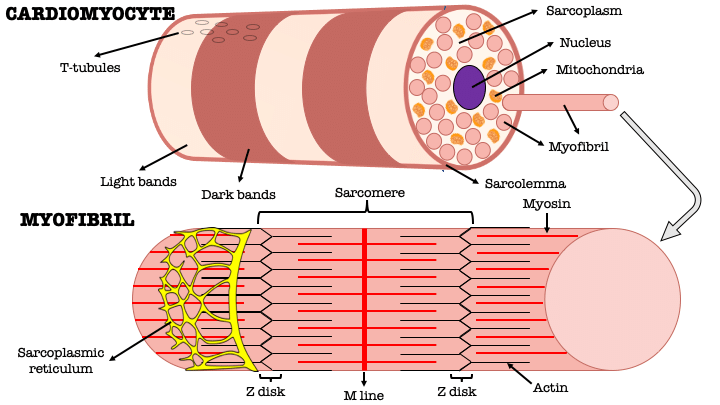
-Each myocyte contains multiple, rod-like cross-banded strands (myofibrils) that run the length of the cell
-Are composed of repeating sarcomeres
-
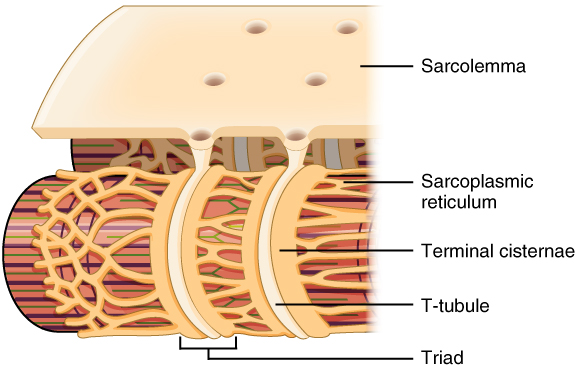
Describe the structure of T-tubules
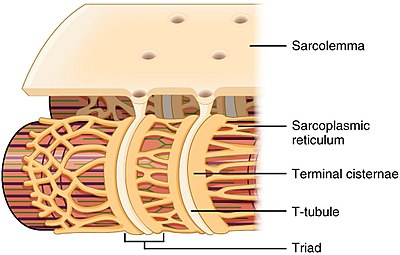
-Are invaginations of the muscle cell membrane (sarcolemma) that penetrate the centre of cardiac muscle cells
-
How do sarcomeres generally cause muscle contraction?
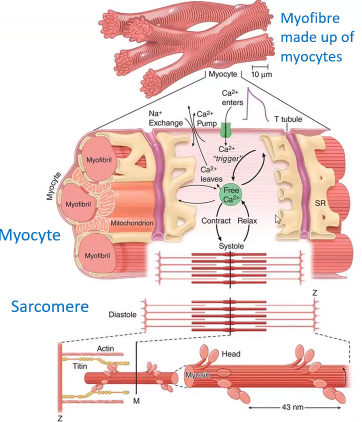
When their component actin and myosin filaments move relative to each other
-
T tubules have what type of channel?
What does this channel ensure?
-Calcium channels
-Ensure calcium is delivered deep into the cell close to the sarcomere
-
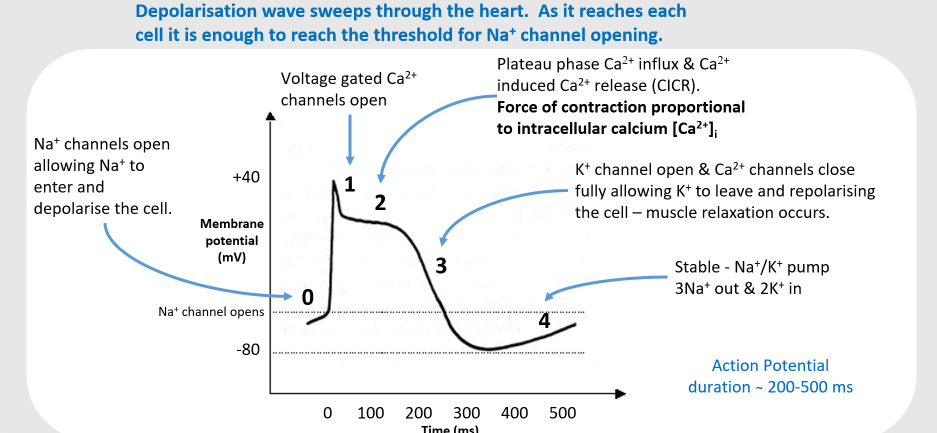
Ca2+ enters via calcium channel that open in response to the wave of depolarization that travels along the sarcolemma where they trigger the release of more.......?
Calcium from the sarcoplasmic reticulum and initiate contraction
-
The varying actin-myosin overlap is shown for _________, when [Ca2+] is maximal, and diastole, when [Ca2+] is minimal.
What is the missing word?
Systole
-
Picture outlining a Rise in intracellular calcium is central to contraction
-
Contraction is determined by INCREASE in [Ca2+]i
Higher increases in Ca2+leads to an increased force of contraction
Intracellular Ca2+ levels increase from.......?
0.1 μM to about 10 μM
-
What does CICR stand for?
Calcium induced calcium release
-
State the intracellular calcuim (Ca2+i) levels during Diastolic, normal systole and maximum systole
Diastolic [Ca2+]I ̴ 0.1 μM
Normal systole [Ca2+]i may rise ̴ 1 μM
Maximum systole [Ca2+]i may rise ̴ 10 μM
-
What is the importance of the CICR (Ca2+ store)? (4)
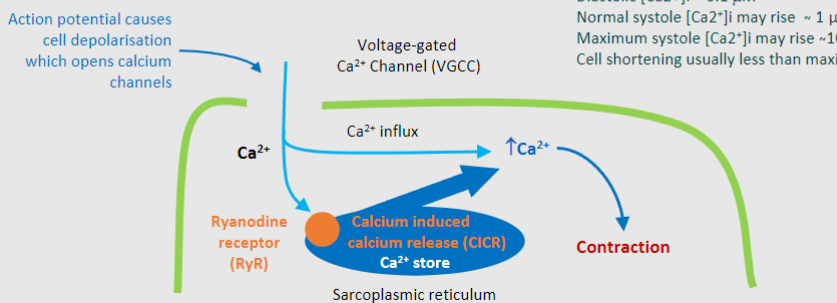
-Amplification of Calcium Signals
-Regulation of Muscle Contraction
-Control of Heartbeat
-Target for Drug Intervention
-
Calcium binds to the _______ Receptor during the process of calcium-induced calcium release (CICR) in muscle cells
What is the missing word?
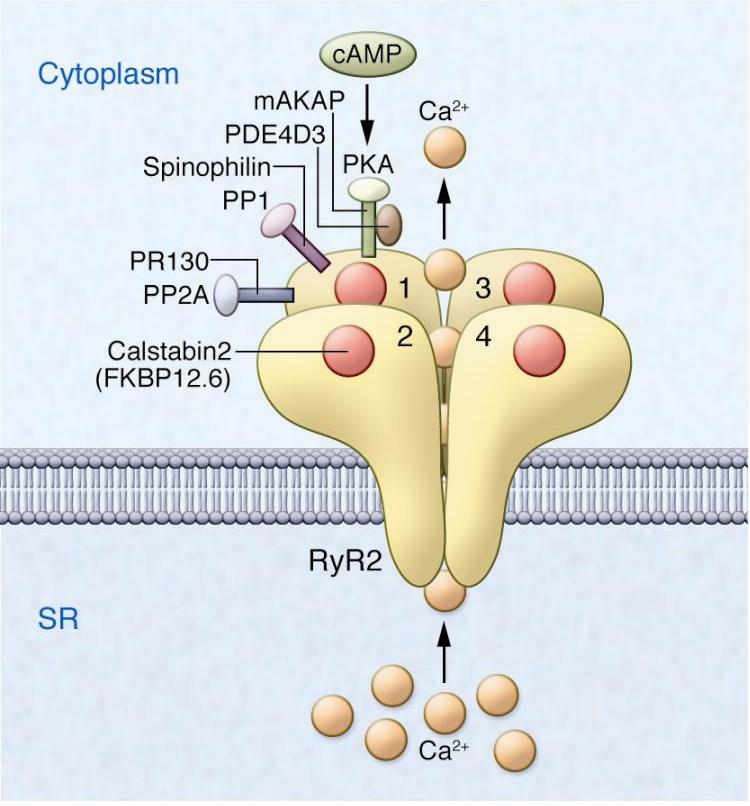
Ryanodine (RyR)
-
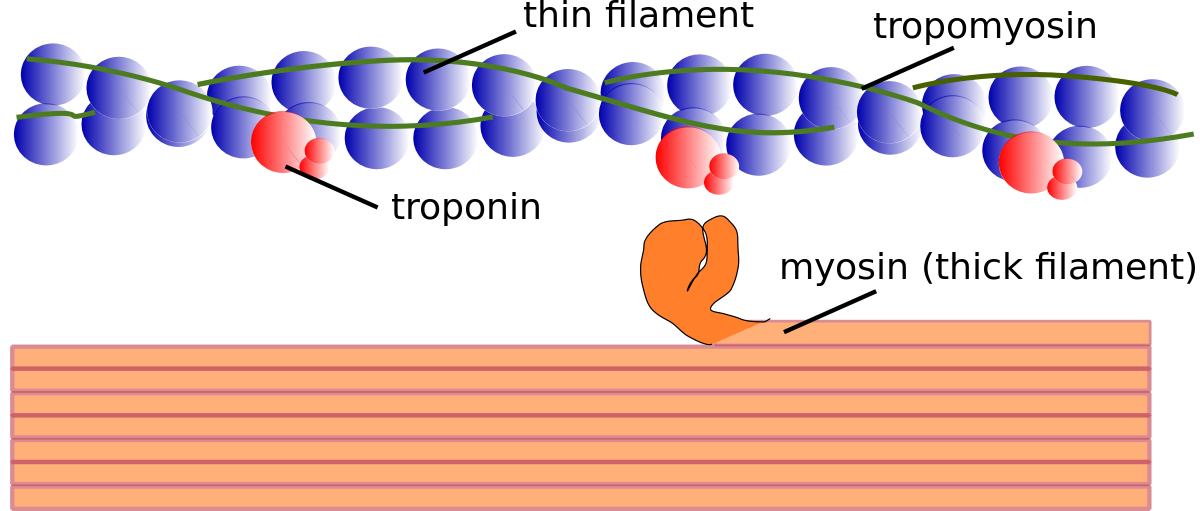
State the Five steps that leads to myosin thick filament binding:
1)Action potential (Na+ ions) depolarises T-tubules & activates VGCCs (Voltage-Gated Calcium Channels) causing Ca2+ influx
2)Ca2+ binds to RyR located on sarcoplasmic reticulum (SR) leads to close association with T-tubules
3)Release of Ca2+ from SR leads to Ca induced Ca release (CICR)
4)Ca2+ binds to troponin, displacement of tropomyosin/troponin complex, exposing active sites on actin
5)Myosin thick filament heads bind to active sites on actin & filaments slide (using ATP)
-
Troponin regulates the conformation of ?
Tropomyosin
-
Troponin is composed of how many regulatory subunits?
What are the names of these subunits and what do they each bind to?
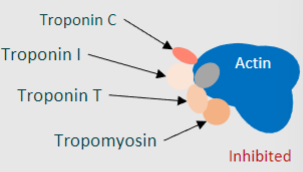
3
-Troponin T ~ binds to tropomyosin
-Troponin I ~ binds to actin filaments
-Troponin C ~ binds Ca2+
-
Binding of Ca2+ to Troponin C (TnC) leads to what 2 things occuring?
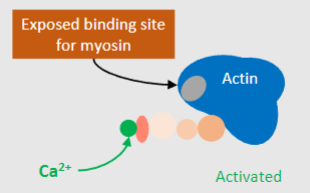
-Conformational changes of tropomyosin
-Exposure of actin binding sites
-
What troponin markers are important blood plasma markers for cell death?
TnI and TnT
-
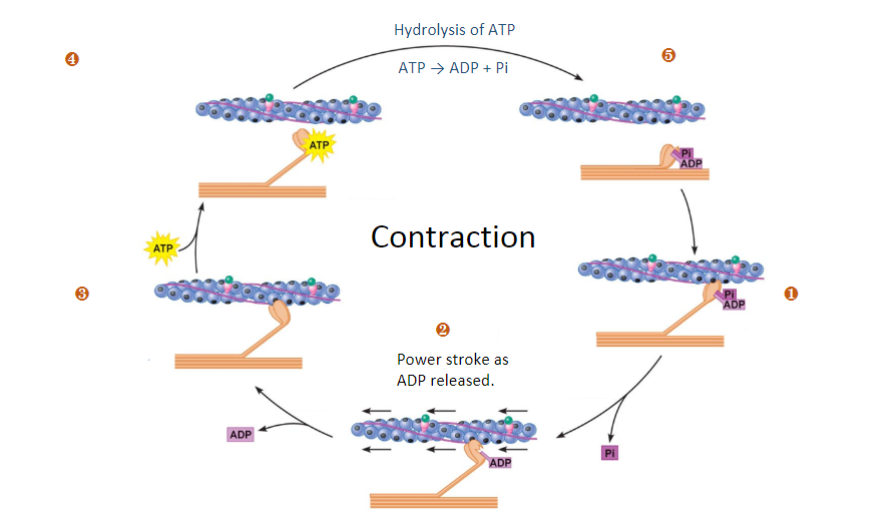
Fill in the blanks (Steps 1-5)
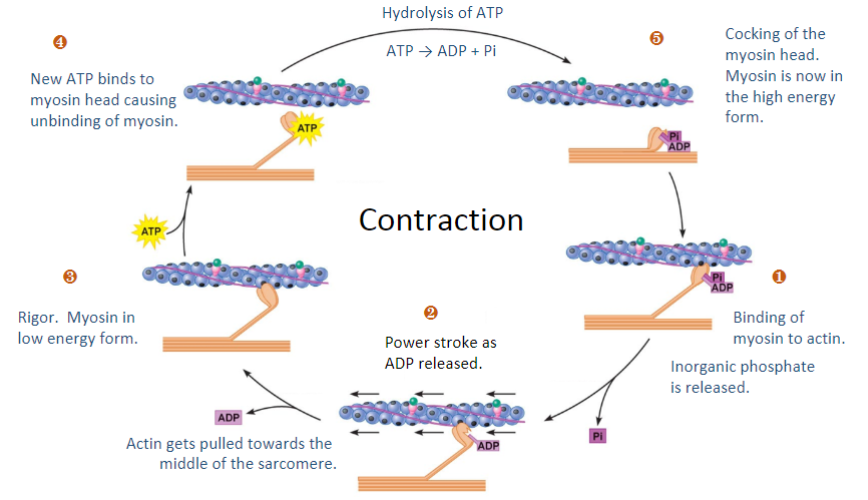
-
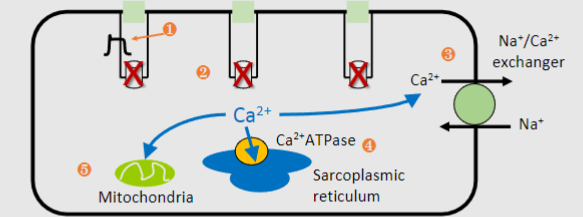
Outline the 5 steps that leads to a decrease in calcium and relaxation (repolarisation)
1)Action potential repolarization: Potassium ions leave the cell, causing repolarization of the T-tubules and closure of VGCCs, reducing calcium influx.
2)Extrusion of calcium from the cell: Calcium is pumped out of the cell, primarily through the Na^+/Ca^2+ exchanger, contributing to around 30% of calcium removal.
3)Uptake of calcium into the sarcoplasmic reticulum (SR): Calcium ions are actively transported back into the SR via the SR membrane Ca^2+-ATPase pump, accounting for approximately 70% of calcium removal.
4)Storage of calcium in the SR for future contractions: Calcium is stored in the SR to be released during the next contraction. Even relaxation requires energy in the form of ATP.
5)Uptake of calcium into mitochondria: Some calcium ions are taken up by mitochondria, helping to maintain calcium homeostasis
-
Reduction in calcium means myosin-actin binding is reduced, preventing contraction
How does this effect the chambers?
The chambers can relax and refill
-
Picture outlining the difference between Starling’s law & contractility (inotropy) - The central idea that with increased stretch there is an increased stroke volume
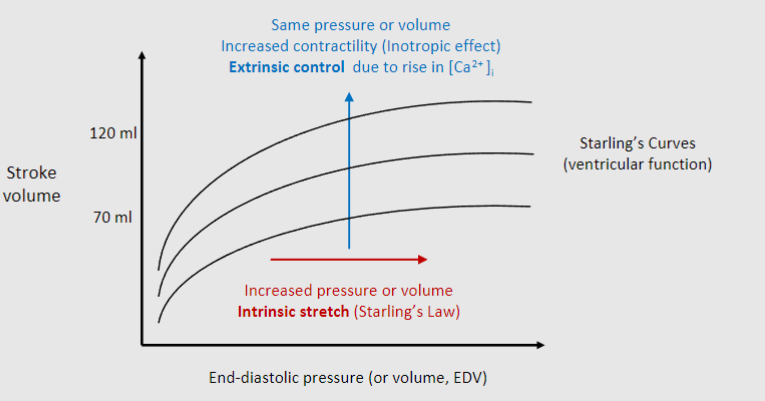
-
To increase contractility, Noradrenaline will act at a receptor by phosphorylating calcium channels and allowing greater Ca2+ influx and higher intracellular levels
What receptor of the sympathetic nervous system does noradrenaline act on?
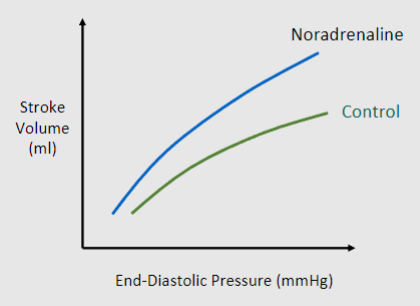
b1 adrenoceptors
-
Drugs are sometimes needed to increase contractility of the heart, mostly to correct acute or chronic heart failure.
In general, these drugs increase intracellular calcium.
In what 2 ways?
1. Increasing voltage gated calcium channel activity (sympathetic mimetic)
2. Reducing Ca2+ extrusion (cardiac glycosides)
-
Where are b1 adrenergic receptors found on the heart specifically?
On the contractile cells of the heart, the atrial and ventricular cells
-
Outline the mechanism on how the binding of noraadrenaline to b1 adrenoreceptors cause increase contractility (Ca2+)
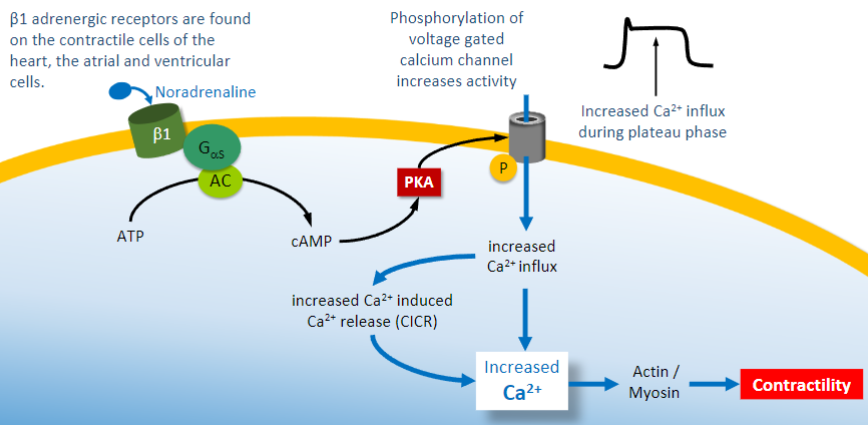
-
Increased protein kinase A (PKA) leads to what 4 things happening?
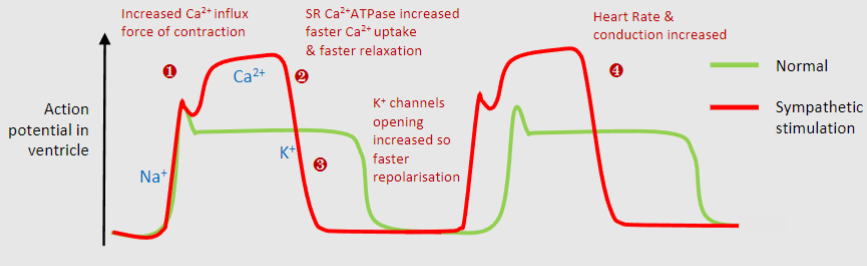
1)Increased Ca2+ channel so higher Ca2+ levels and greater contraction
2)Increased sarcoplasmic reticulum Ca2+ATPase, so uptake of Ca2+ into storage by SR allowing faster relaxation
3)Increased K+ channel opening so faster repolarisation and shorter action potential, leads to faster heart rate
4)Overall stronger faster contractions but same diastolic time to allow for filling with blood & coronary perfusion
-
What does Digoxin do generally?
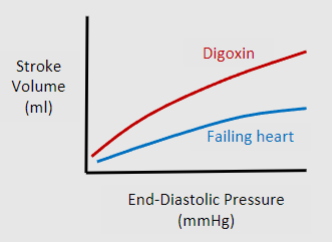
Increases contractility by reducing Ca2+ extrusion
-
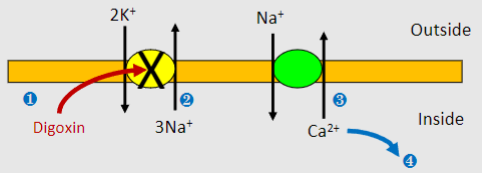
What is the mechanism of action of Digoxin? (4)
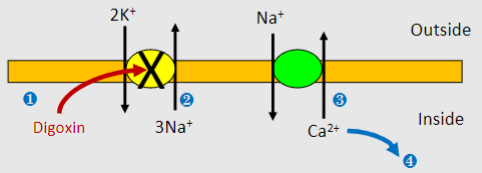
1)Digoxin inhibits Na+/K+ ATPase
2)Build up of intracellular Na+ lowers concentration gradient (which normally powers Na+/Ca2+ exchanger)
3)Less Ca2+ expulsion by Na+/Ca2+ exchanger
4)More Ca2+ uptake into stores and greater CICR
-
What is Digoxin derived from?

Foxgloves
-
Digoxin is used for heart treatment, what are some preferred first line treatments?
-ACE inhibitors
-Beta blockers
-
What is Digoxin used to treat (3)
-Atrial fibrillation
-Atrial flutter
-Congestive heart failure
-
What are some side effects of Digoxin? (8)
-Unusual tiredness and fatigue
-Anxiety
-Hallucinations
-Visual disturbances
-Nausea or vomiting
-Arrhythmias
-Electrolyte imbalances
-Can be worsened by other medications
-
What are 3 other inotropic agents?
-Dobutamine and dopamine
-Glucagon
-Amrinone
-
What is Dobutamine and dopamine? And when are they used?
-b1 adrenoceptor stimulants
-May be used in acute heart failure
-
Where does glucagon act? What does it do? And when is it used?
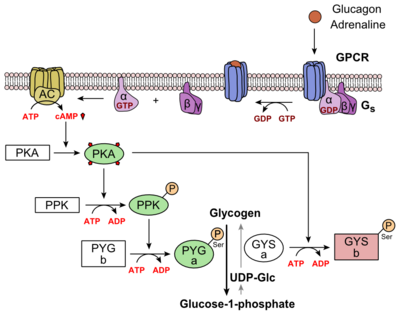
-Acts at G protein coupled receptor
-Stimulates GS pathway, increases cAMP and PKA activity-Used in patients with acute heart failure who are taking β-blockers
-
What is Amrinone? When is it used?
-A phosphodiester inhibitor
-Only used in severe cases e.g. those waiting for heart transplants
-
How does the action of Amrinone lead to inotropic activity of the heart? (3)
-Type III phosphodiesterase (PDE3) is heart specific, and converts cAMP into AMP
-This normally reduces cAMP and decreases PKA activity as a result, limiting contractility
-Inhibition by Amrinone leads to a build up of cAMP causing increased activation of PKA which phosphorylates Ca2+ channels and increases Ca2+ influx.

