-
Hydrocarbons
Molecules consisting only of carbon and hydrogen
-
functional group
A molecular group attached to a hydrocarbon that confers chemical properties or reactivities. Examples include hydroxyl (– OH), carboxylic acid (– COOH) and amino groups (– NH2).
-
Hydroxyl
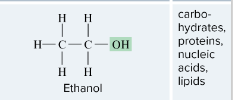
--OH
-
Carbonyl
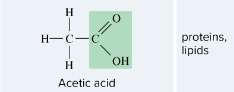
-
Amino
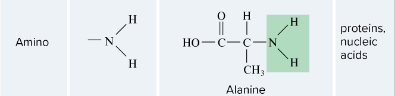
-
Sulfhydril
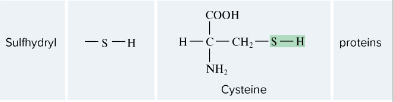
-
Phosphate
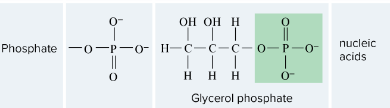
-
Methyl
-
Isomers
One of a group of molecules identical in atomic composition but differing in structural arrangement; for example, glucose and fructose.
-
Traditional Grouping of Biological Molecules
Carbohydrates, nucleic acids, proteins, and lipids
-
Polymers
A molecule composed of many similar or identical molecular subunits, called monomers
starch is a polymer of glucose.
-
monomer
The smallest chemical subunit of a polymer. The monosaccharide α-glucose is the monomer found in plant starch, a polysaccharide.
-
dehydration reaction
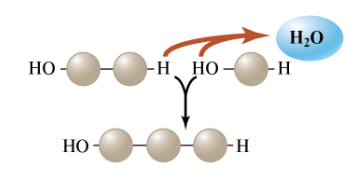
A type of chemical reaction in which two molecules join to form one larger molecule, simultaneously splitting out a molecule of water; one molecule is stripped of a hydrogen atom, and another is stripped of a hydroxyl group (– OH), resulting in the joining of the two molecules, while the H and – OH released may combine to form a water molecule.
-
Hydrolysis reaction
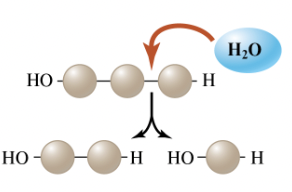
A type of chemical reaction in which a molecule of water is added instead of removed. A hydrogen attom is attached to one subunit and a hydroxyl group to the other, breaking the covalent bond and joining the subunits.
-
Carbohydrates
Loosely defined group of molecules that all contain carbon, hydrogen, and oxygen in the molar ratio 1:2:1
Well suited for energy storage
-
Monosaccharides
Simplest of carbohydrates. "simple sugars". Contain as few as 3 carbon atoms. Central role in energy storage monosaccharides have 6 carbon atoms.
-
disaccharide
A carbohydrate formed of two simple sugar molecules bonded covalently.
-
polysaccharide
A carbohydrate composed of many monosaccharide sugar subunits linked together in a long chain;
examples are glycogen, starch, and cellulose.
-
starch
An insoluble polymer of glucose; the chief food storage substance of plants.
-
cellulose
The chief constituent of the cell wall in all green plants, some algae, and a few other organisms; an insoluble complex carbohydrate formed of microfibrils of glucose molecules.
-
glycogen
Animal starch; a complex branched polysaccharide that serves as a food reserve in animals, bacteria, and fungi.
-
chitin
A tough, resistant, nitrogen-containing polysaccharide that forms the cell walls of certain fungi, the exoskeleton of arthropods, and the epidermal cuticle of other surface structures of certain other invertebrates.
-
deoxyribonucleic acid (DNA)
The genetic material of all organisms; composed of two complementary chains of nucleotides wound in a double helix.
Functions to store information.
-
ribonucleic acid (RNA)
A class of nucleic acids characterized by the presence of the sugar ribose and the pyrimidine uracil; includes mRNA, tRNA, and rRNA.
-
Functions of RNA
Carries genetic information in form of mRNA
Part of the ribsome in form of ribsomal RNA (rRNA)
Carries amino acids in the form of transfer RNA (tRNA)
Regulates the process of gene expression.
-
messenger RNA (mRNA)
The RNA transcribed from structural genes; RNA molecules complementary to a portion of one strand of DNA, which are translated by the ribosomes to form protein.
-
nucleotide
A single unit of nucleic acid, composed of a phosphate, a 5-carbon sugar (either ribose or deoxyribose), and a purine or a pyrimidine.
-
Purines found in DNA
Adenine (A)
Guanine (G)
Cytosine (C)
Thymine (T)
-
Purines found in RNA
Adenine (A)
Guanine (G)
Cytosine (C)
Uracil (U)
-
nucleic acid
A nucleotide polymer; chief types are deoxyribonucleic acid (DNA), which is double-stranded, and ribonucleic acid (RNA), which is typically single-stranded.
-
double helix
The structure of DNA, in which two complementary polynucleotide strands coil around a common helical axis.
-
Base-pairing rules in DNA
Adenine-thymine (A-T)
Cytosine-guanine (C-G)
-
Base-pairing rules in RNA
Adenine-uracil (A-U)
Cytosine-guanine (C-G)
-
complementary
Describes genetic information in which each nucleotide base has a complementary partner with which it forms a base-pair.
-
ribosomal RNA (rRNA)
A class of RNA molecules found, together with characteristic proteins, in ribosomes; transcribed from the DNA of the nucleolus.
-
transfer RNA (tRNA)
A class of small RNAs (about 80 nucleotides) with two functional sites; at one site, an “activating enzyme” adds a specific amino acid, while the other site carries the nucleotide triplet (anticodon) specific for that amino acid.
-
adenosine triphosphate (ATP)
A nucleotide consisting of adenine, ribose sugar, and three phosphate groups; ATP is the energy currency of cellular metabolism in all organisms.
-
nicotinamide adenine dinucleotide (NAD)
A molecule that becomes reduced (to NADH) as it carries high-energy electrons from oxidized molecules and delivers them to ATP-producing pathways in the cell.
-
flavin adenine dinucleotide (FAD, FADH2)
A cofactor that acts as a soluble (not membrane-bound) electron carrier (can be reversibly oxidized and reduced).
-
Seven categories of Proteins
1. Enzyme catalysis
2. Defense
3. Transport
4. Support
5. Motion
6. Regulation
7. Storage
-
Protein function: Enzyme catalysis
Enzymes are biological catalysts that facilitate specific chemical reactions.
Most enzymes are 3-D globular proteins that fit snugly around the molecules they act on. This facilitates chemical reactions by stressing particular chemical bonds.
-
Protein function: Defense
Globular proteins use their shapes to "recognize" foreign microbes and cancer cells.
Cell-surface receptors form the core of the body's endocrine and immune systems.
-
Protein function: Transport
A variety of globular proteins transport small molecules and ions.
Membrane transport proteins help move ions and molecules across the membrane.
-
Protein function: support
Protein fibers play structural roles.
-
Protein function: Motion
Muscles contract thru the sliding motion of two kinds of protein filaments: actin and myosin
Contractile proteins also play key roles in the cell's cytoskeleton and in moving materials within cells
-
Protein function: Regulation
Small proteins called hormones serve as intercellular messengers in animals.
Also play many regulatory roles within the cell's cytoskeleton and in moving materials within cells.
-
Protein function: Storage
Calcium and iron are stored in the body by binding as ions to storage proteins.

