Biological Molecules (The Living Cell) UNFINISHED
Learning Objectives By the end of this lecture, students will be able to: • Be familiar with the make-up of molecules essential for life • Identify and understand the differences between each of the essential biomolecules • Appreciate structure-function relationships and how this can lead to dysfunction
-
Biological molecules are based on what molecule?
Biological molecules are carbon-based (except for somesmall inorganic molecules)
-
What is the composition of the cell (%s of water, inorganic ions, small organic molecules and macromolecules)
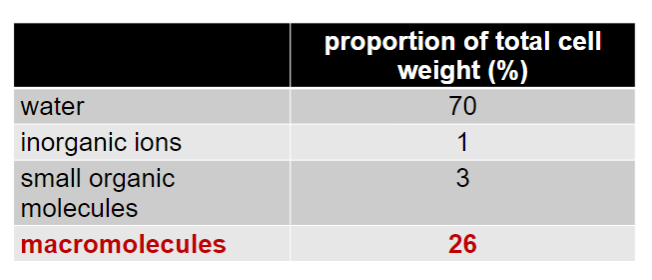
-
State some information about carbohydrates:
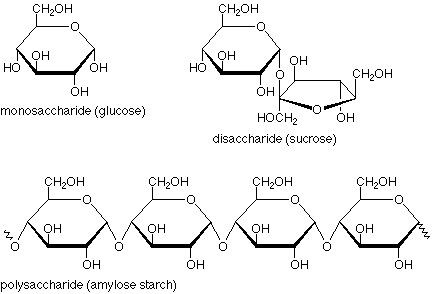
• General formula: (CH2O)n• Most common organiccompound on earth.• Function as energy storage,fuel, metabolite, and structuralelement
-
State some information about monosaccharides:
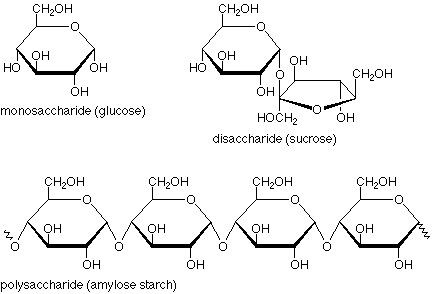
•All have the formula (CH2O)n• Two kinds of monosaccharides:1) Ketone based → ketose2) Aldehyde based → aldose• Number of C-atoms determinesname:▫ triose, tetrose, pentose, hexose,heptose• They have chiral centres= enantiomers
-
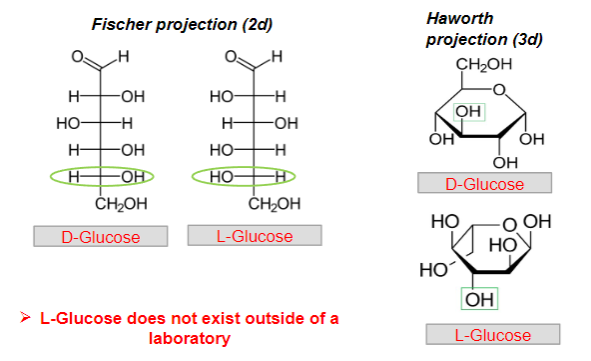
D- vs L-monomers of monosaccharides, what are the differences?
D-Monomers:
In a D-monomer, the hydroxyl (OH) group on the chiral carbon farthest from the carbonyl group is on the right side.
Think of "D" as "right."
L-Monomers:
In an L-monomer, the hydroxyl (OH) group on the chiral carbon farthest from the carbonyl group is on the left side.
Think of "L" as "left."
-
Picture demonstrating D- vs L-monomers of monosaccharides:
-
Can L-glucose exist naturally?
NO
It cannot exist outside a laboratory
-
State some information about glucose:
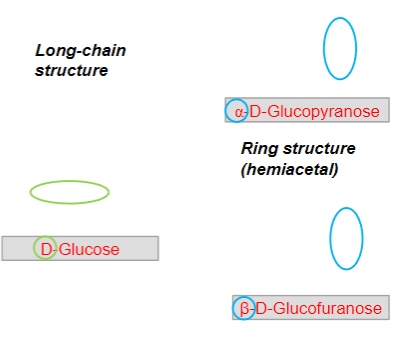
• An aldose• 6 C-atoms: hexose• D-configuration:asymmetric C-atommost distant fromthe aldehyde/ketonegroup• Can exist in long-chain and ringstructure
-
Disaccharides are formed via what type of reaction?
Condensation reaction
-
Name the 3 types of polysaccharides and what type of bonding corresponds to each of them
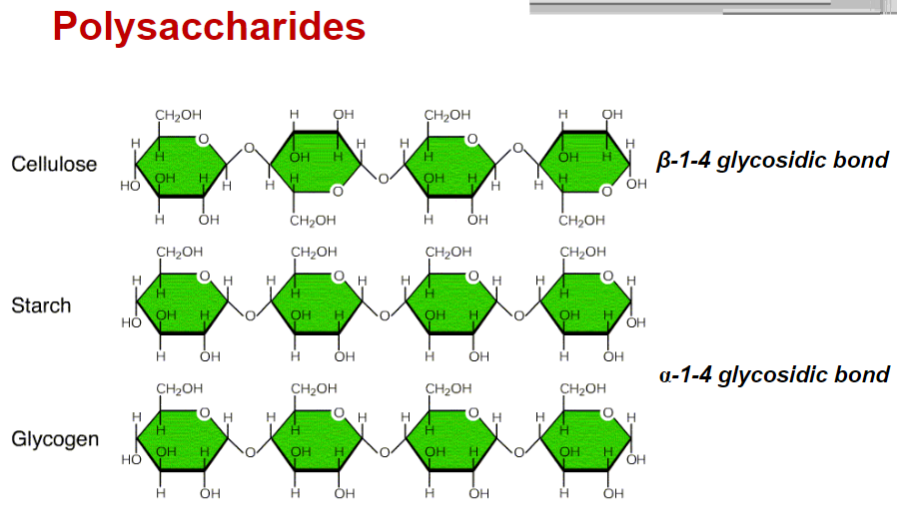
-
State, some information about glycogen:
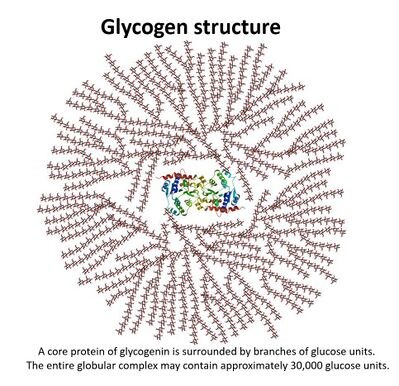
•An aldose• 6 C-atoms: hexose

