-
How do prokaryotes regulate protein translation?
mRNAs have a 6 nucleotide Shine-Dalgarno sequence upstream of the AUG start codon.
-
What are the mechanisms in which they can regulate proteins?
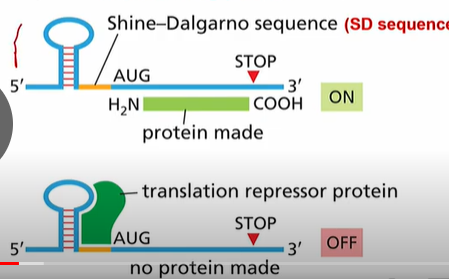
1. An RNA binding protein that blocks access to the SD sequence
2. Temperature Regulated RNA structure: stem loop RNA structure blocks the SD sequence. When temperature increases, the loop unwinds.
3. Riboswitch, changes structure of RNA blocking the SD
4. Antisense RNA: Base pairs with mRNA and block SD
-
Do prokaryotes or eukaryotes have Shine-Dalgarno sequences?
Only Prokaryotes
-
How is ferritin regulated?
Since in eukaryotes there are no SD sequences they just block the AUG start codon. During iron starvation, cytosolic aconitase binds to the ferritin mRNA and no translation occurs. When there is excess iron, iron binds to cytosolic aconitase and releases it allowing the start codon to be accessed and translation can occur.
-
Which is eukaryotic and which is prokaryotic. Antisense RNA and microRNA(miRNA)?
Antisense RNA is prokaryotic and microRNA is eukaryotic
-
What role does eIF2 play in eukaryotic translation?
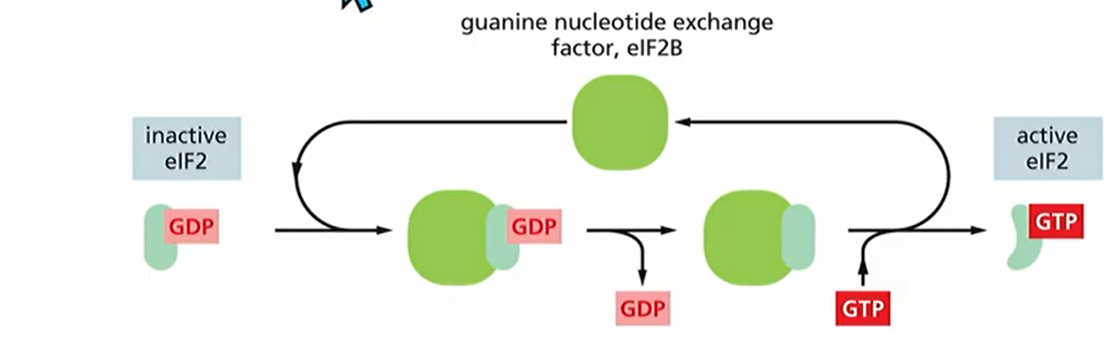
It forms a complex with GTP and recruits initiator tRNA to small ribosomal subunit. It then binds to 5' end and searches for start codon. When it finds start codon GTP gets hydrolyzed to GTP and causes conformational change in eIF2. Causing eIF2 bound with GDP to be released now there's no GTP for translation to work. Reactivation requires eIF2B which is guanine exchange factor (GEF). Meaning it changes GDP for GTP this is regulated by phosphorylation. Basically if eIF2 is phosphorylated and eIF2B comes, it just doesn't work.
-
What are the steps that proteins undergo in order to become functional?
1. Fold properly to adopt their 3D structure
2. Be covalently modified with chemical groups like sugars and phosphates
3. Proteins interact with other proteins and small molecules.
-
What type of amino acids are buried in the interior core of the protein?
Hydrophobic ones
-
How do proteins fold themselves?
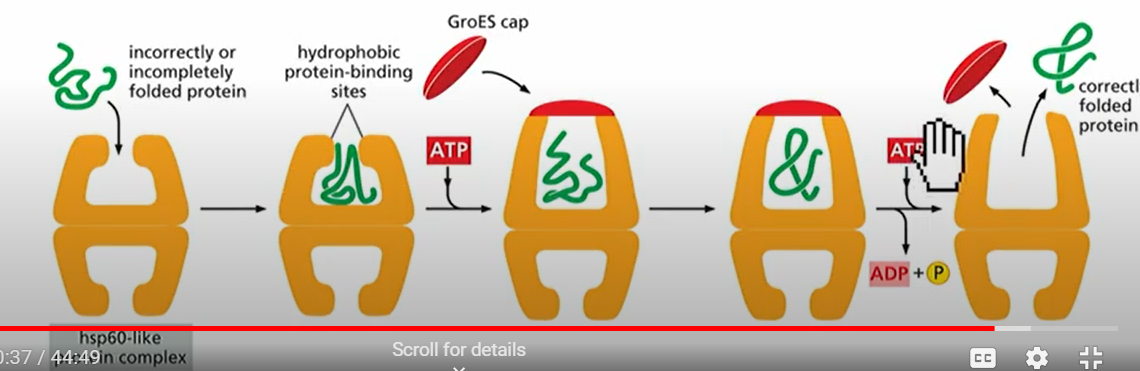
After they emerge from the ribosomes, but some need help from chaperone proteins called heat-shock proteins(Hsp). Named this way because MORE are made at high temperatures. Hsp70 and Hsp60 assist in protein folding by interacting with hydrophobic residue in misfolded proteins and use ATP hydrolysis.
-
what are the two things that help stop misfolded proteins?
1. Chaperone proteins help it become functional proteins
2. Proteasome which marks proteins with hydrophobic residues sticking out for degradation.
Compete with each other.
Also longer time to fold, longer chance of being degraded
-

A