-
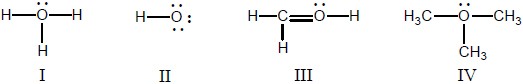
In which structure(s) below does the oxygen have a formal charge of +1?
I, III, and IV
-
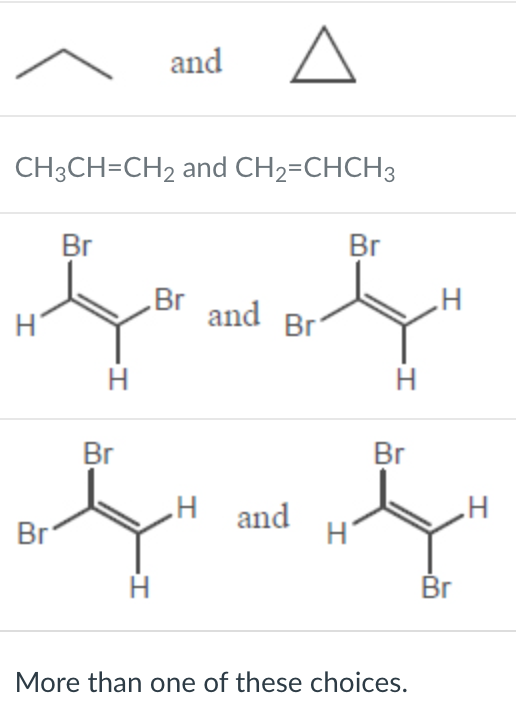
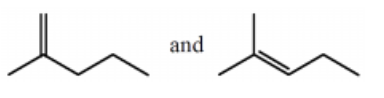
Which of the following represent a pair of constitutional isomers?
More than one of these choices.
-
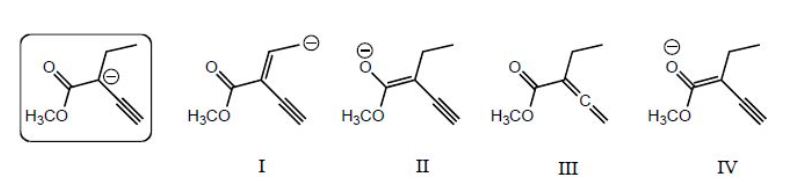
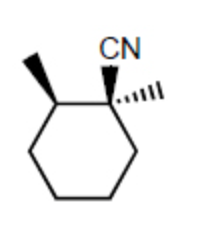
Which of the following species is/are a resonance form(s) of the species in the box?
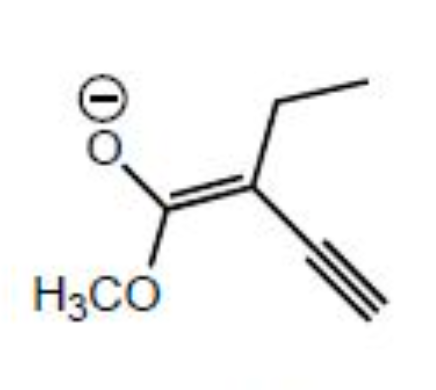
II
-
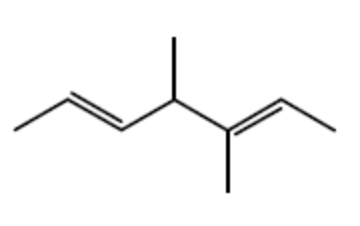
How many s-sp2 bonds are there in the following substance?
3
-
Which compound would have the highest boiling point?
CH3CH2CH2CH2CH2CH3
CH3CH2OCH2CH2CH3
CH3CH2CH2CH2CH2OH
CH3CH2OCH(CH3) 2
CH3OCH2CH2CH2CH3
CH3CH2CH2CH2CH2OH
-
For the functional group(s) on the following molecule what characteristic IR absorption(s) would be expected (ignoring C-H absorptions)? a peak around 1700 cm-1
a peak around 3300 cm-1
a peak around 1650 cm-1
a peak around 2250 cm-1
a peak around 2250 cm-1
-
The IR stretching frequency can be predicted to occur at the highest frequency for which of these bonds?
C–H
C–F
C–Cl
C–Br
C–I
C-H
-
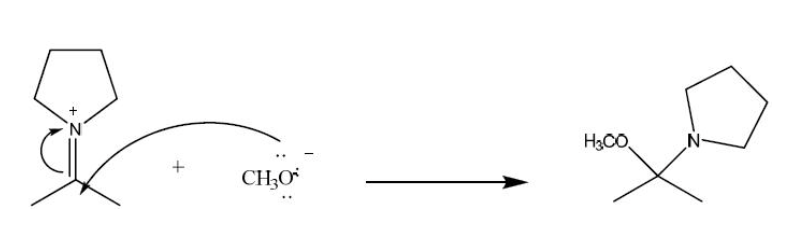
In the following reaction which chemical species is acting like a nucleophile?
CH3O-
-
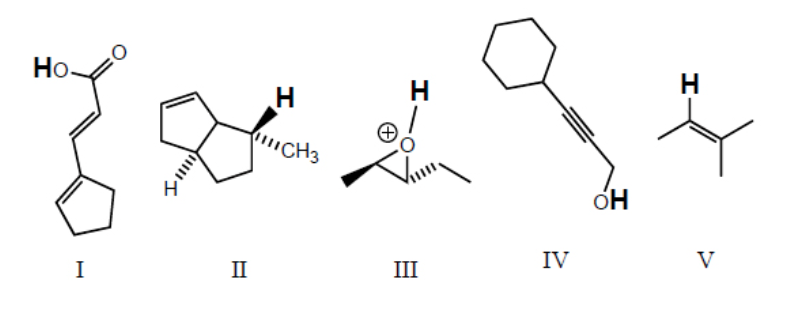
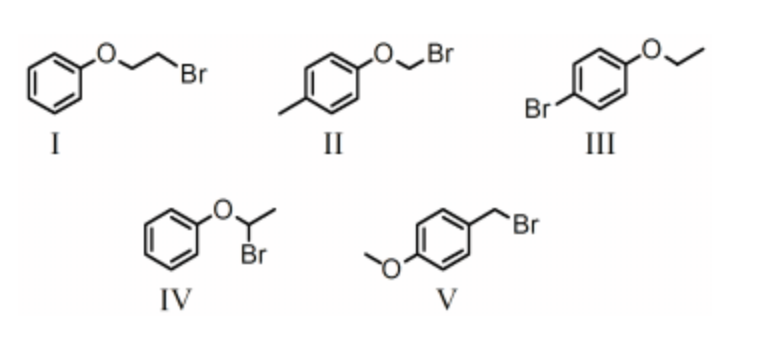
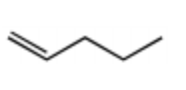
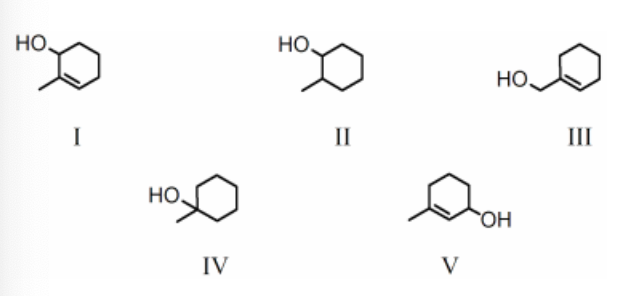
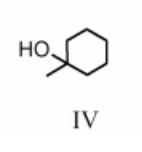
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic.
III > I > IV > V > II
-
Which acid-base reaction would not take place as written?
CH3Li + CH3CH2CH2CH2NH2 ⎯⎯⎯⎯→ CH4 + CH3CH2CH2CH2NHLi
CH3C≡CH + NaOCH3 ⎯⎯⎯⎯→ HC≡CNa + CH3OH
HC≡CNa + H2O ⎯⎯⎯⎯→ HC≡CH + NaOH
CH3OH + NaNH2 ⎯⎯⎯⎯→ CH3ONa + NH3
CH3CO2H + CH3ONa ⎯⎯⎯⎯→ CH3CO2Na + CH3OH
CH3C≡CH + NaOCH3 ⎯⎯⎯⎯→ HC≡CNa + CH3OH
-
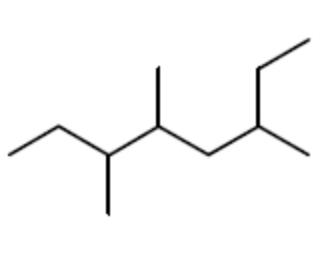
The IUPAC name for this is:
6-Ethyl-3,4-dimethylheptane
2-Ethyl-4,5-dimethylheptane
3,4,6-Trimethyloctane
3,5,6-Trimethyloctane
2-(1-Methylpropyl)-4-methylhexane
3,4,6-Trimethyloctane
-
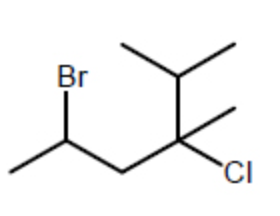
Provide a name for the following compound:
2-Bromo-4-chloro-4-isopropylpentane
4-Bromo-2-chloro-2-isopropylpentane
5-Bromo-3-chloro-2,3-dimethylhexane
2-Bromo-4-chloro-4,5-dimethylhexane
2-(2-Bromopropyl)-2-chloro-3-methylbutane
5-Bromo-3-chloro-2,3-dimethylhexane
-
![A correct name for the following compound is:4-bromo-3,8-dimethylbicyclo[5.2.2]nonane 3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane 4-bromo-3,8-dimethylbicyclo[5.2.1]decane 7-bromo-2,6-dimethylbicyclo[5.2.0]nonane 4-bromo-3,8-dimethylbicyclo[5.2.0]nonane](/flashcards/cardimage2/ea2abe85/551/7551416_face.png)
A correct name for the following compound is:
4-bromo-3,8-dimethylbicyclo[5.2.2]nonane
3,8-dimethyl-4-bromo-bicyclo[5.2.0]nonane
4-bromo-3,8-dimethylbicyclo[5.2.1]decane
7-bromo-2,6-dimethylbicyclo[5.2.0]nonane
4-bromo-3,8-dimethylbicyclo[5.2.0]nonane
4-bromo-3,8-dimethylbicyclo[5.2.0]nonane
-
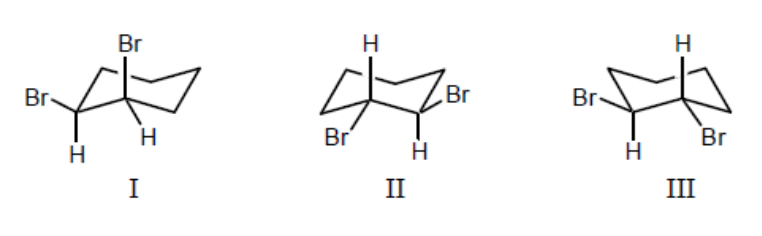
trans-1,2-Dibromocyclohexane is represented by structure(s):
II and III
-
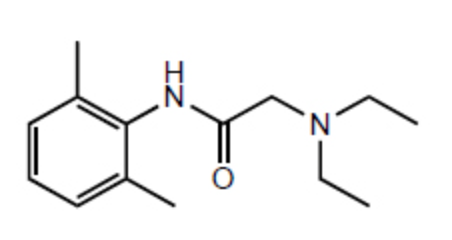
What is the index of hydrogen deficiency (or degree of unsaturation) for the drug lidocain?
3
4
5
6
7
5
-
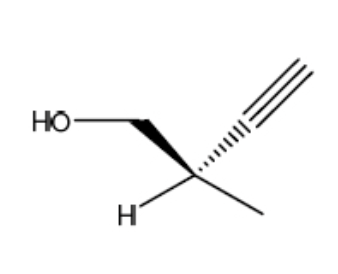
What is IUPAC name of the following compound?
(R) -2-methyl-3-butyn-1-ol
(S) -2-methyl-3-butyn-1-ol
(R) -2-methyl-1-butyn-3-ol
(S) -2-methyl-1-butyn-3-ol
(R) -2-methyl-3-butyn-1-ol
-
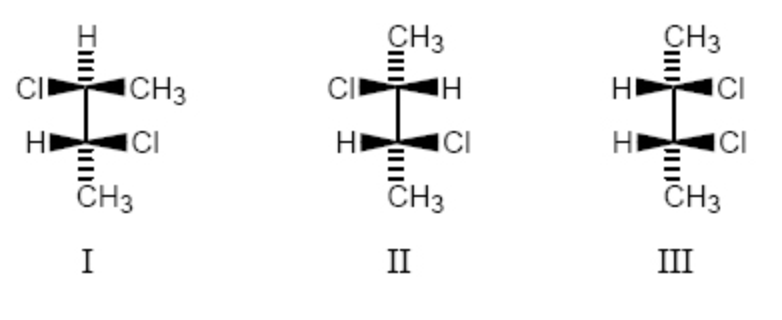
Which of the following is (are) meso compound(s) ?
I and III
-
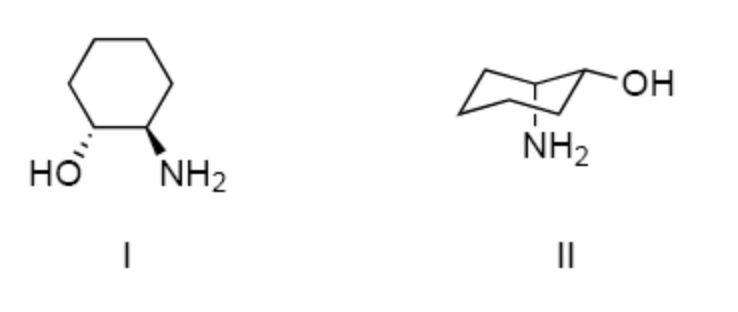
I and II are:
constitutional isomers.
enantiomers.
identical.
diastereomers.
not isomeric.
diastereomers
-
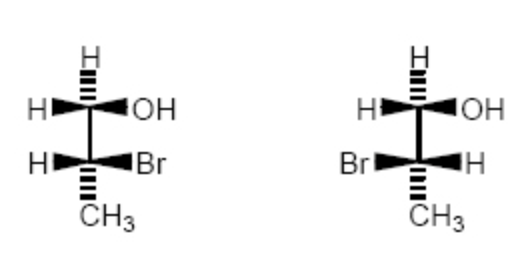
The molecules shown are:
constitutional isomers.
enantiomers.
diastereomers.
identical.
None of these choices.
enantiomers
-
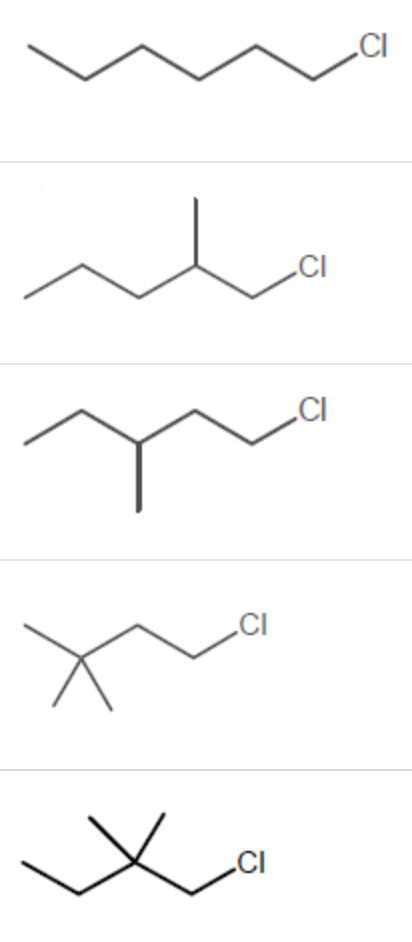
Which alkyl chloride, though primary, is essentially unreactive in SN2 reactions?
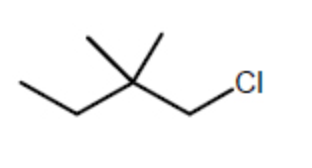
-
Which alkyl halide would you expect to react most slowly when heated in aqueous solution?
(CH3)3C-F
(CH3)3C-Cl
(CH3)3C-Br
(CH3)3C-I
All of these choices would react at the same rate.
(CH3)3C-F
-
Which SN2 reaction will occur most rapidly in aqueous acetone solution? Assume concentrations and temperature are the same in each instance.
HO- + CH3-Cl ⎯⎯⎯⎯→ CH3OH + Cl-
HO- + CH3CH2-Cl ⎯⎯⎯⎯→ CH3CH2OH + Cl-
HO- + (CH3)2CH-Cl ⎯⎯⎯⎯→ (CH3)2CHOH +Cl-
HO- + (CH3)3C-Cl ⎯⎯⎯⎯→ (CH3)3COH + Cl-
HO- + (CH3)3CCH2-Cl ⎯⎯⎯⎯→ (CH3)3CCH2OH + Cl-
HO- + CH3-Cl ⎯⎯⎯⎯→ CH3OH + Cl-
-
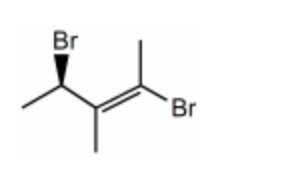
Name the following compound:
(S,E)-2,4-dibromo-3-methylpent-2-ene
(R,Z)-2,4-dibromo-3-methylpent-2-ene
(R,E)-2,4-dibromo-3-methylpent-3-ene
(S,E)-2,4-dibromo-3-methylpent-3-ene
(R,E)-2,4-dibromo-3-methylpent-2-ene
(R,E)-2,4-dibromo-3-methylpent-2-ene
-
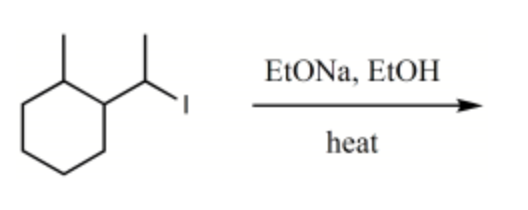
What is the major product for the following reaction?
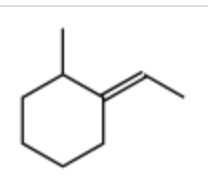
-
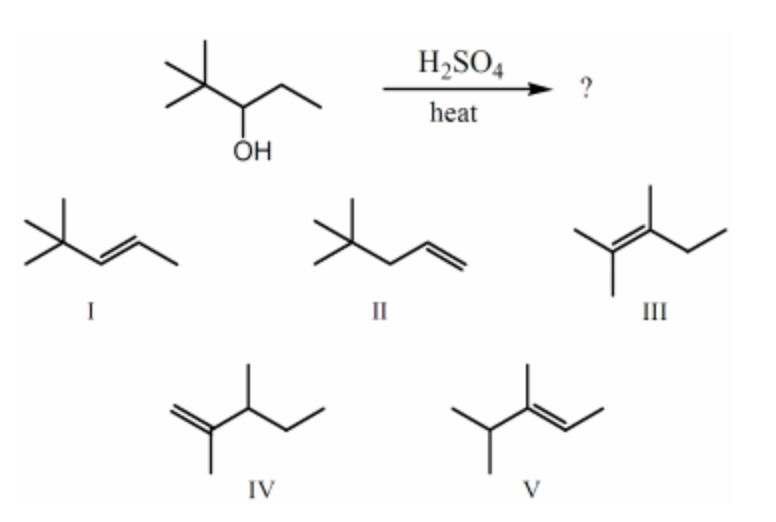
Which alkene would you expect to be the major product of the following dehydration?
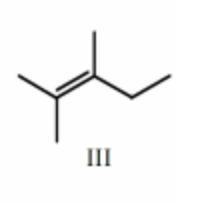
III
-
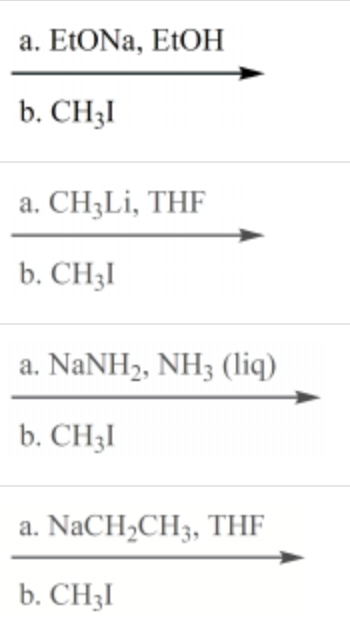
Which reaction conditions would not yield 2-butyne from 1-propyne?
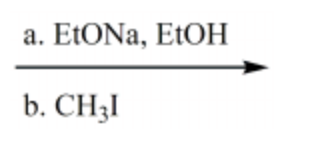
-
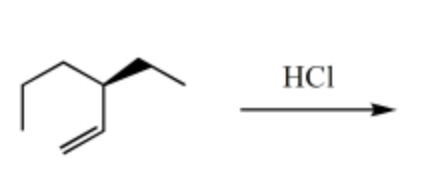
What is the major product for the following reaction?
-
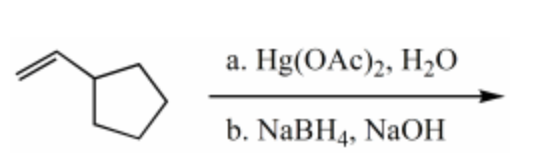
What is the major product for the following reaction?
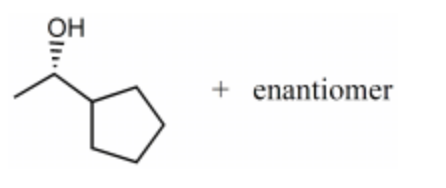
-
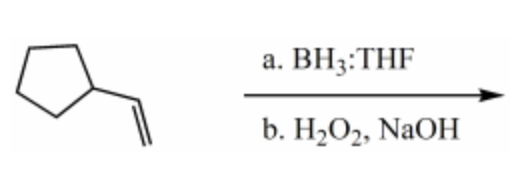
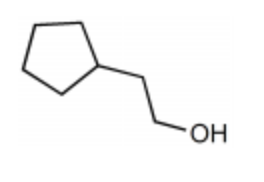
What is the major product for the following reaction?
-
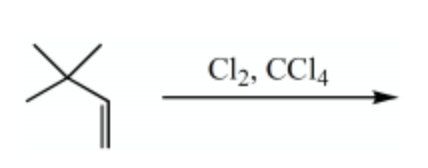
What is the major product for the following reaction?
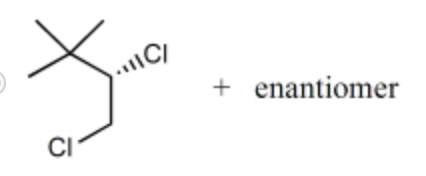
-
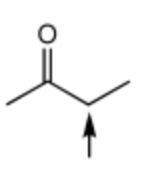
Which one of the following best represents the predicted approximate chemical shift and coupling for the hydrogen(s) indicated with the arrow?
1.00 ppm, quartet
2.40 ppm, singlet
2.40 ppm, quartet
3.00 ppm, quartet
2.40 ppm, triplet
2.40 ppm, quartet
-
For the following compound how many different signals would you see in the proton NMR? (Assume that you can see them all.)
4
-
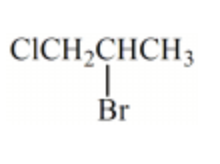
How many chemically distinct 1H NMR signals are there in the following compound?
4
-
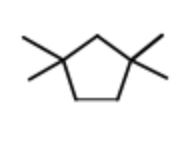
Consider the expected 1H NMR spectrum of 1,1,3,3-tetramethylcyclopentane. Which of the following is likely to be observed?
7 signals: all singlets
7 signals: 4 singlets, 3 doublets
3 signals: all singlets
3 signals: 1 singlet, 2 doublets
3 signals: 2 singlets,1 doublet
3 signals: all singlets
-
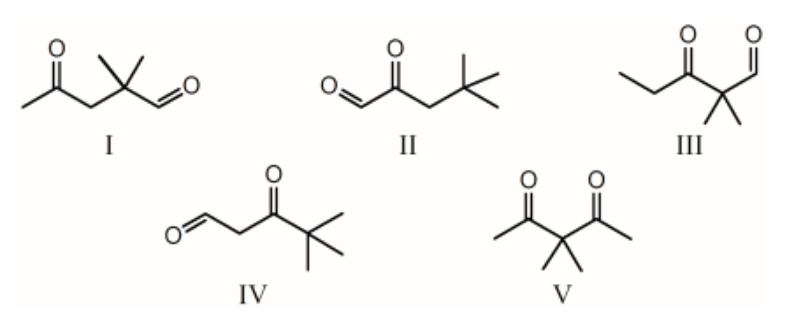
The 1H NMR spectrum of which of the compounds below, all of formula C7H12O2, would consist of three singlets only?
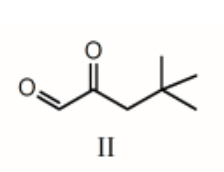
II
-
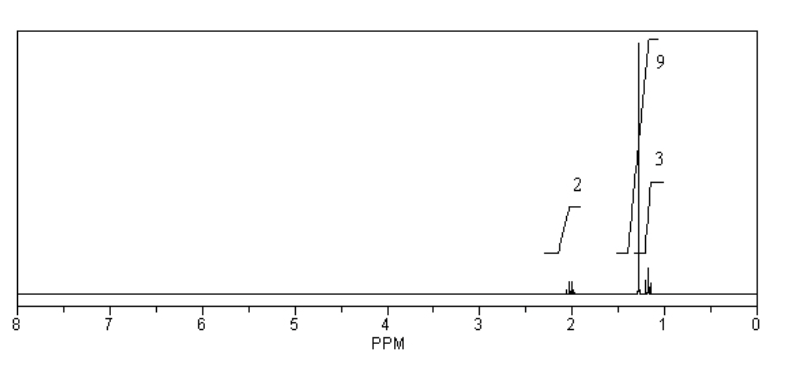
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C8H14? Relative integration is shown.
-
A compound with the molecular formula C8H9BrO gave the following 1H NMR spectrum:triplet, δ 1.4quartet, δ 3.9multiplet, δ 7.0 (4H)There was no evidence of an -OH band in the IR spectrum. A possible structure for the compound is:
III
-
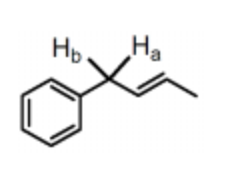
In the structure shown, Ha and Hb are classified as:
homotopic protons.
vicinal protons.
enantiotopic protons.
diastereotopic protons.
isomeric protons.
enantiotopic protons.
-
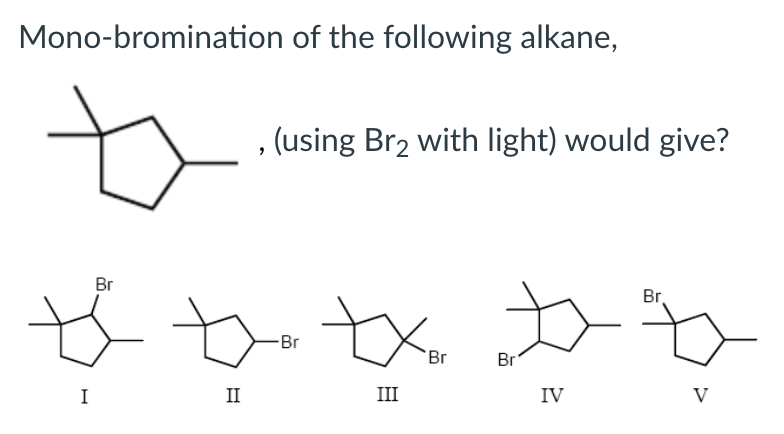
Mono-bromination of the following alkane (using Br2 with light) would give?
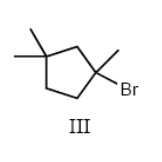
III
-
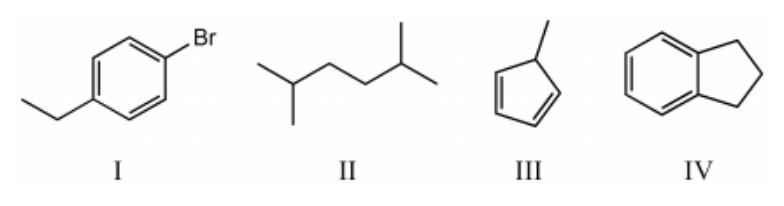
Which compound below would give rise to 4 signals in the proton NMR spectrum and 6 signals in the carbon NMR spectrum? (Assume you can separate and see all peaks.)
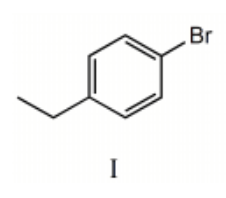
I
-
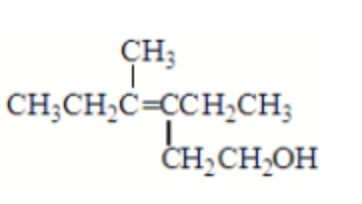
What is the correct IUPAC name for the following compound?
3-methyl-4-ethyl-3-hexen-6-ol
4-ethyl-3-methyl-3,6-hexenol
3-ethyl-4-methyl-3-hexen-1-ol
3-methyl-4-(2-
hydroxyethyl)-3-hexene
3-(2-hydroxyethyl)- 3-methyl-3-hexene
3-ethyl-4-methyl-3-hexen-1-ol
-
Which compound would have the greatest solubility in water?
Diethyl ether
Methyl propyl ether
1-Butanol
1,2-Butanediol
Pentane
1,2-Butanediol
-
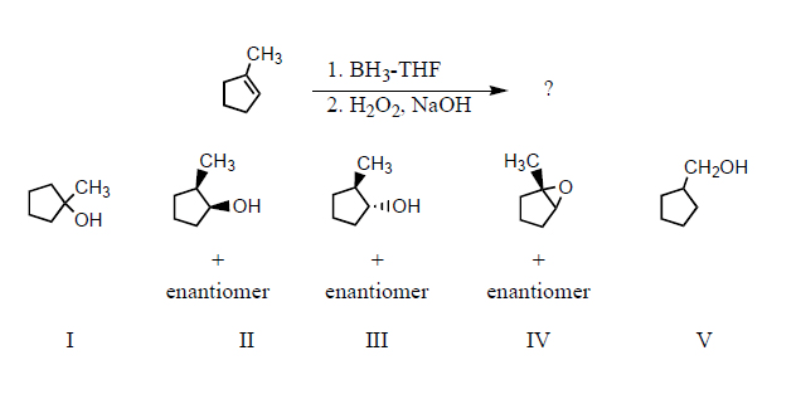
Which product(s) would you expect to obtain from the following sequence of reactions?
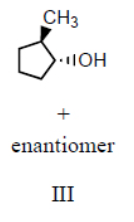
III
-
The reaction between 1-pentanol and HBr to yield 1-bromopentanol is probably:
an SN1-type reaction involving the protonated alcohol as the substrate.
an SN2-type reaction involving the protonated alcohol as the substrate.
an E1-type reaction involving the protonated alcohol as the substrate.
an E2-type reaction involving the protonated alcohol as the substrate.
an SN2-type reaction involving the protonated alcohol as the substrate.
-
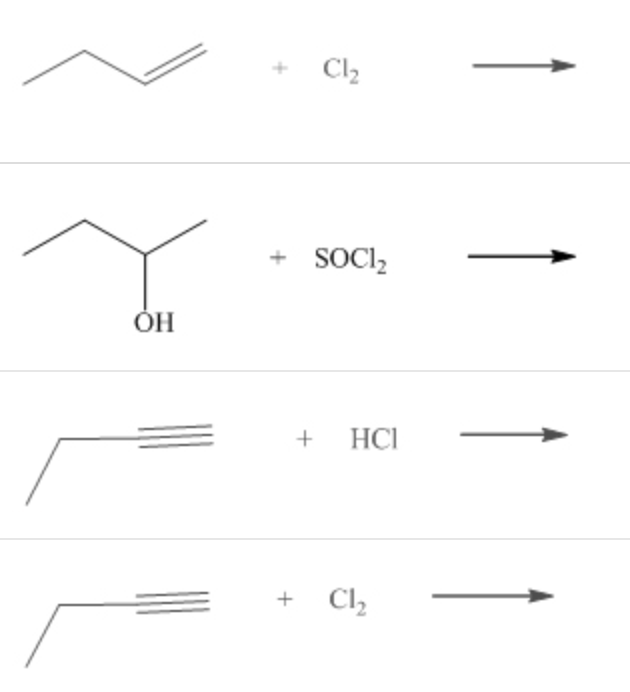
Which of the following could be used to synthesize 2-chlorobutane?

-
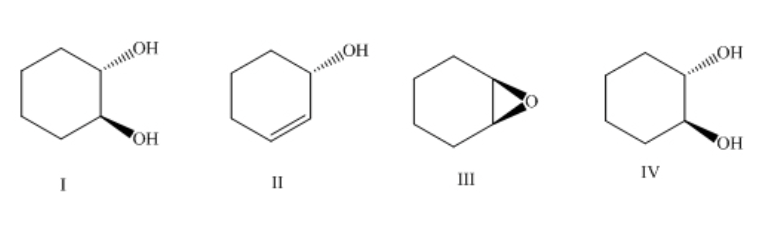
If trans-2-bromocyclohexanol is treated with sodium hydroxide what is the major product?
-
The most stable conformation of cis-1-tert-butyl-2-methylcyclohexane is the one in which:
the tert-butyl group is axial and the methyl group is equatorial in a chair conformation.
the methyl group is axial and the tert-butyl group is equatorial in a chair conformation.
both groups are axial in a chair conformation.
both groups are equatorial in a chair conformation.
the twist boat conformation is adopted.
The methyl group is axial and the tert-butyl group is equatorial in a chair conformation.
-
What is the major product formed after treating the following molecule with excess H2 in the presence of Pd/C catalyst?
cis-2-penten-5-ol
1-pentanol
pentane
none of these choices
1-pentanol
-
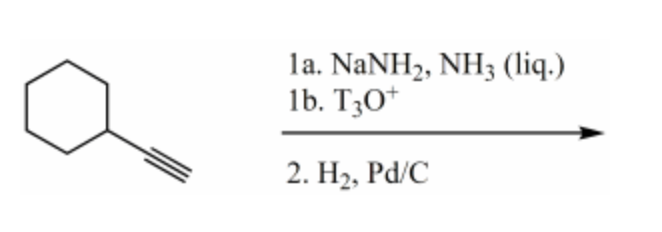
Which would be the major product of the following reaction sequence?
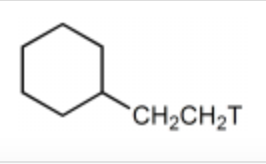
-
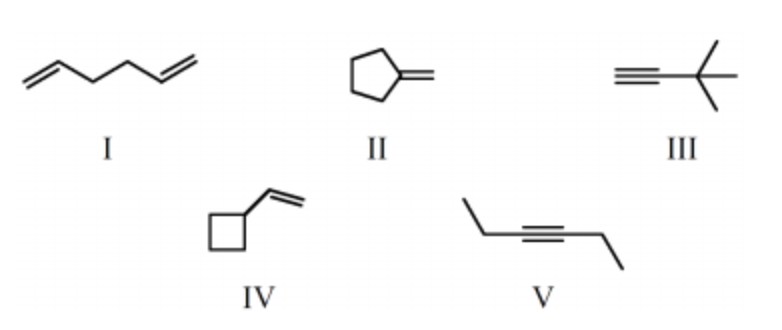
A compound, C6H10, which reacts with 2 mol of hydrogen over a platinum catalyst and which shows an IR absorption band at approximately 3300 cm-1 could be:
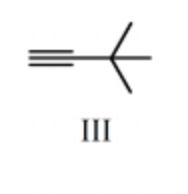
III
-
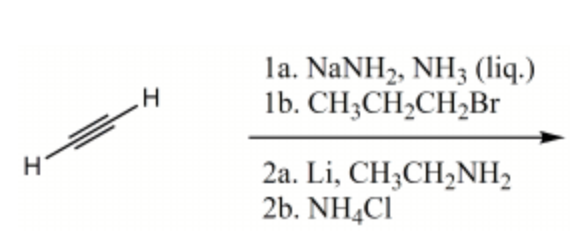
What is the major product for the following reaction sequence?
-
Which of the following could be used as the basis for a simple test that would distinguish between 1-pentyne and pentane?
IR examination
Br2/CCl4
KMnO4/H2O
Two of these choices.
All of these choices.
All of these choices.
-
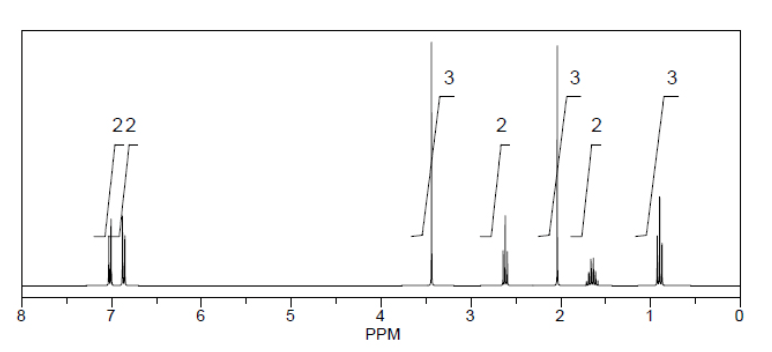
What is the structure of the compound in the following 1H-NMR spectrum with the molecular formula C12H17NO and a characteristic IR stretch near 1700 cm-1 and a characteristic peak in the 13C-NMR at 170 ppm? Relative integration is shown.
-
In general, when the addition of an unsymmetrical electrophilic reagent to an unsymmetrical alkene forms the product predicted by Markovnikov's rule, that occurs because:
the product is statistically favored.
steric hindrance favors its formation.
it is formed via the more/most stable carbocation.
it is the more/most stable product.
All of these choices are reasons.
it is formed via the more/most stable carbocation.
-

Consider the reaction of 2-chloro-2-methylpentane with sodium iodide.Assuming no other changes, how would it affect the rate if one simultaneously doubled the concentration of 2-chloro-2-methylpentane and sodium iodide?
It would quadruple the rate.
No effect.
It would double the rate.
It would increase the rate five times.
It would triple the rate.
It would double the rate.
-
Which of the following alkyl bromide isomers would most readily undergo an SN2 reaction?
4-bromo-1-butene
3-bromo-1-butene
All will react at the same rate.
bromocyclobutane
1-bromo-1-butene
4-bromo-1-butene
-
Which of the following solvents will best promote a nucleophilic reaction between NaCN and 1-bromopropane?
HF(aq)
running with no solvent
MeCN
H2O/MeOH mixture
H2O
MeCN
-
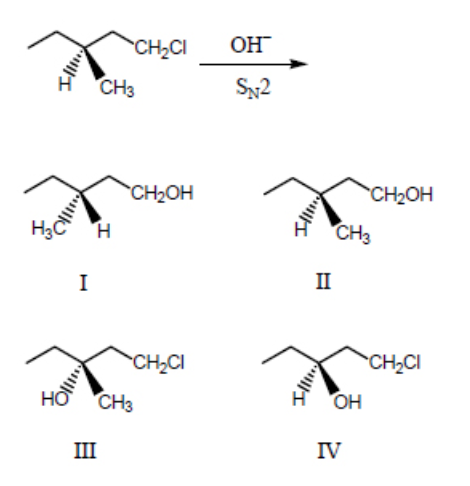
What would be the major product of the following reaction?
-
SN1 reactions of the following type:Nu:- + R-X ⎯⎯⎯⎯→ R-Nu + :X-are favored
by use of a weak nucleophile.
by increasing the polarity of the solvent.
by the use of tertiary substrates (as opposed to primary or secondary substrates) and by use of a weak nucleophile.
by the use of tertiary substrates (as opposed to primary or secondary substrates).
by increasing the concentration of the nucleophile.
by the use of tertiary substrates (as opposed to primary or secondary substrates) and by use of a weak nucleophile.
-
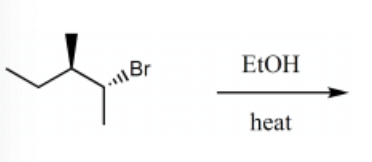
The product(s) for the following reaction would mainly be dictated by which mechanism?
E1
SN1
SN2
E2
E1
-
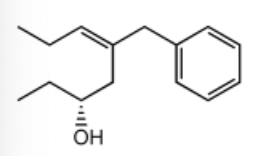
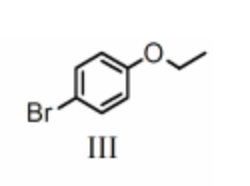
Name the following compound:
(R,Z)-5-benzyloct-5-en-3-ol
(R,E)-5-benzyloct-3-en-6-ol
(R,Z)-5-phenyloct-5-en-3-ol
(R,E)-5-phenyloct-5-en-3-ol
(R,E)-5-benzyloct-5-en-3-ol
(R,Z)-5-benzyloct-5-en-3-ol
-
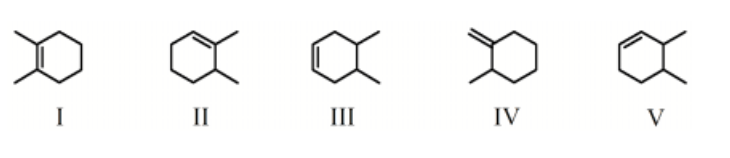
Which molecule would have the lowest heat of hydrogenation?
-
Which product(s) would be produced by acid-catalyzed dehydration of 2-methyl-2-pentanol?
-
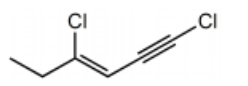
Which structure represents (Z)-1,4-dichlorohex-3-en-1-yne?
-
Treating 1-methylcyclohexene with H3O+ would yield primarily which of these?
-
Cyclohexene reacts with bromine to yield 1,2-dibromocyclohexane. Molecules of the product would:
be a meso compound and, in its most stable conformation, it would have one bromine atom equatorial and one axial.
be a pair of diastereomers and, in their most stable conformation, one would have the bromines equatorial and axial, and the other would have the bromines equatorial and equatorial.
be a racemic form and, in their most stable conformation, they would have one bromine atom equatorial and one axial.
be a racemic form and, in their most stable conformation, they would have both bromine atoms equatorial.
be a meso compound and, in its most stable conformation, it would have both bromine atoms equatorial.
be a racemic form and, in their most stable conformation, they would have both bromine atoms equatorial.
-
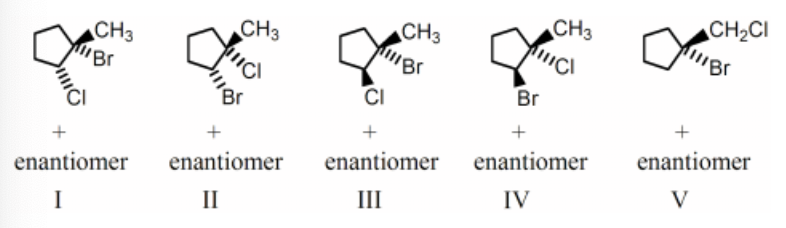
The reaction of BrCl (bromine monochloride) with 1-methylcyclopentene will produce which of the following as the predominant product:
IV
-
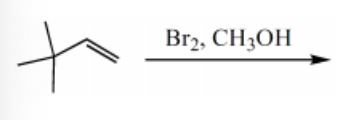
What is the major product for the following reaction?
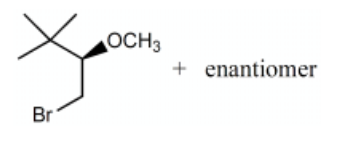
-
Which reagent(s) given below could be used to synthesize cis-1,2-cycloheptanediol from cycloheptene?
H2SO4, heat
KMnO4, OH−, 5oC
None of these choices.
KMnO4, H3O+, 75oC
All of these choices.
KMnO4, OH−, 5oC
-
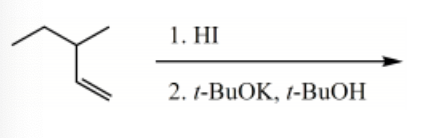
What is the major product of the following reaction sequence?
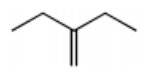
-
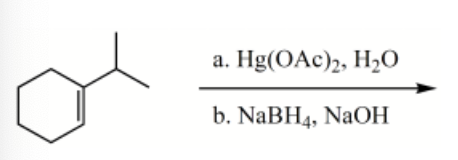
What is the major product for the following reaction?
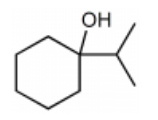
-
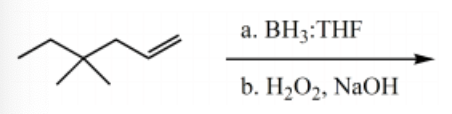
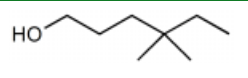
What is the major product for the following reaction?
-
Which ion is the strongest nucleophile in an aprotic solvent such as dimethylsulfoxide?
F-
I-
Cl-
Br-
All of these choices are equal.
F-
-
Which of the following is not a good leaving group?
I−
Cl−
C2H5O−
CH3CO2−
All of these choices are good leaving groups.
C2H5O−
-
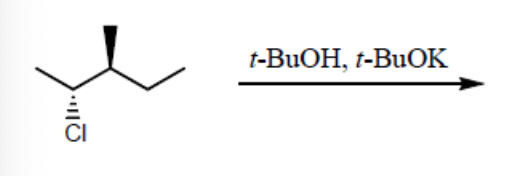
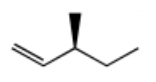
What is the major product for the following reaction?
-
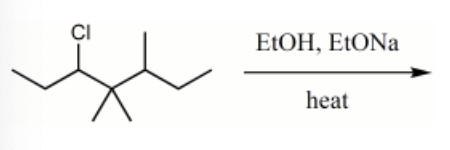
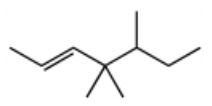
What is the major product for the following reaction?
-
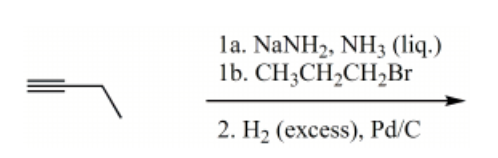
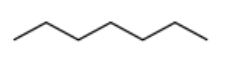
What is the major product for the following reaction sequence?
-
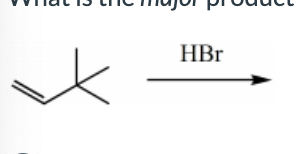
What is the major product for the following reaction?
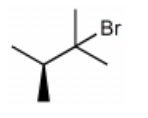
-
What is the simplest alkane, i.e., the one with the smallest molecular weight, which possesses primary, secondary and tertiary carbon atoms?
2-Methylpropane
2-Methylbutane
2-Methylpentane
3-Methylpentane
2,2-Dimethylbutane
2-Methylbutane
-
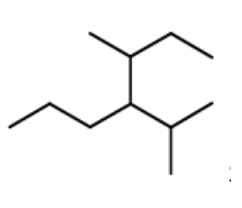
An IUPAC name for this is:
5-Methyl-4-(1-methylpropyl)hexane
2-Methyl-3-(1-methylpropyl)hexane
2-Methyl-3-(2-methylpropyl)hexane
3-Methyl-4-(1-methylethyl)heptane
5-Methyl-4-(1-methylethyl)heptane
3-Methyl-4-(1-methylethyl)heptane
-
Neglecting stereochemistry, which of these common group names is ambiguous, i.e., does not refer to one specific group?
Butyl
sec-Butyl
tert-Pentyl
Neopentyl
sec-Pentyl
sec-Pentyl
-
How many compounds with the formula C7H16 (heptanes) contain a single 3° carbon atom?
4
-
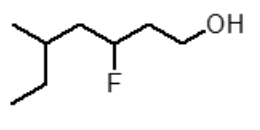
What is the correct IUPAC name for the following compound?
5-Ethyl-3-fluorohexanol
5-Ethyl-3-fluoro-1-hexanol
2-Ethyl-4-fluoro-6-hexanol
3-Fluoro-5-methyl-7-heptanol
3-Fluoro-5-methyl-1-heptanol
3-Fluoro-5-methyl-1-heptanol
-
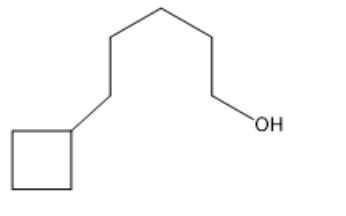
The correct IUPAC name for the following compound is:
5-(1-pentanolyl)-cyclobutane
5-cyclobutyl-1-pentanol
1-cyclobutyl-5-pentanol
1-(5-pentanolyl)-cyclobutane
none of these choices
5-cyclobutyl-1-pentanol
-
![Which of the following is bicyclo[3.2.2]nonane?](/flashcards/cardimage2/4c1ef65f/551/7551490_face.png)
Which of the following is bicyclo[3.2.2]nonane?
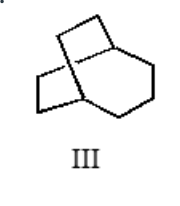
III
-
![What is the correct name of the following compound?1-Chlorobicyclo[4.1.1]octane2-Chlorobicyclo[4.1.0]octane2-Chlorobicyclo[4.1.1]octane2-Chlorobicyclo[4.1.1]heptane5-Chlorobicyclo[4.1.1]octane](/flashcards/cardimage2/dee856b1/551/7551491_face.png)
What is the correct name of the following compound?
1-Chlorobicyclo[4.1.1]octane
2-Chlorobicyclo[4.1.0]octane
2-Chlorobicyclo[4.1.1]octane
2-Chlorobicyclo[4.1.1]heptane
5-Chlorobicyclo[4.1.1]octane
2-Chlorobicyclo[4.1.1]octane
-
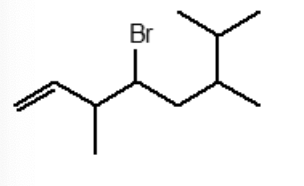
A correct IUPAC name for the following compound is:
3,6,7-trimethyl-4-bromo-1-octene
4-bromo-3-methyl-6-isopropyl-1-heptene
4-bromo-3,6,7-trimethyl-1-octene
4-bromo-6-isopropyl-3-methyl-1-heptene
4-bromo-6-isopropyl-3,6-dimethyl-1-hexene
4-bromo-3,6,7-trimethyl-1-octene
-
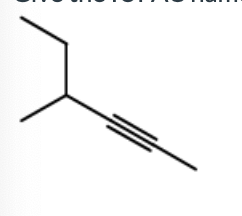
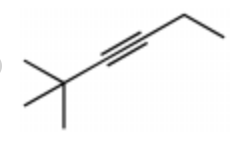
Give the IUPAC name for this
3-Methyl-4-hexyne
4-Methyl-2-hexyne
2-Ethyl-3-pentyne
4-Ethyl-2-pentyne
3-Methyl-2-hexyne
4-Methyl-2-hexyne
-
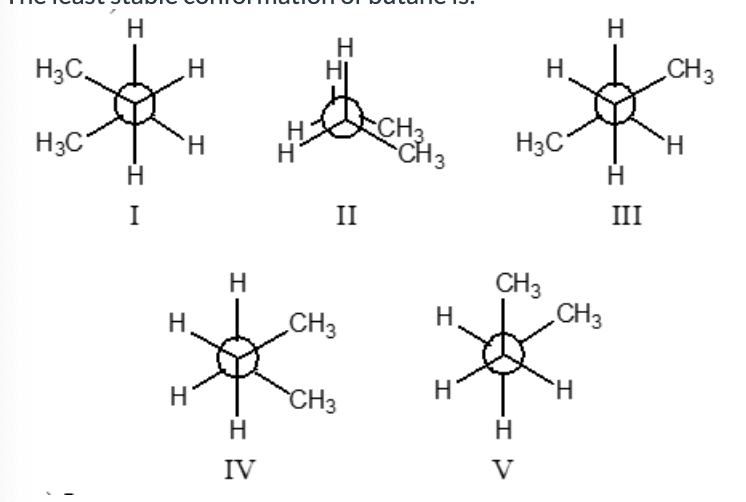
The least stable conformation of butane is:
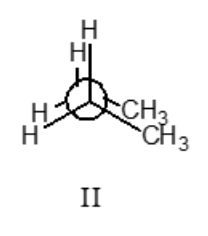
II
-
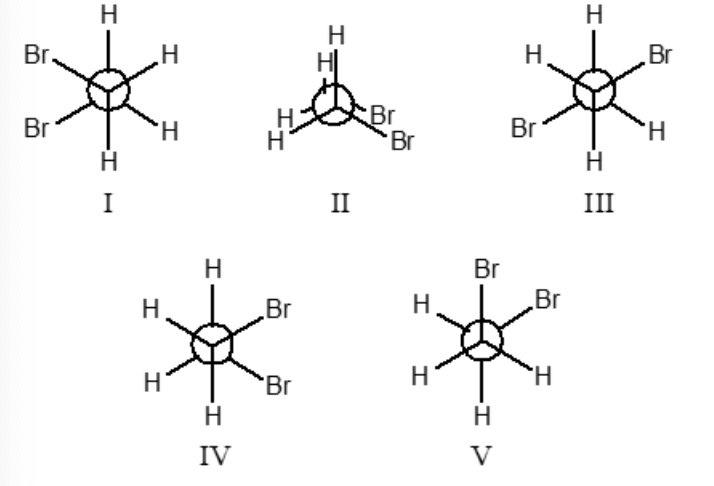
Which conformation(s) of 1,2-dibromoethane does not illustrate one or more gauche interactions?
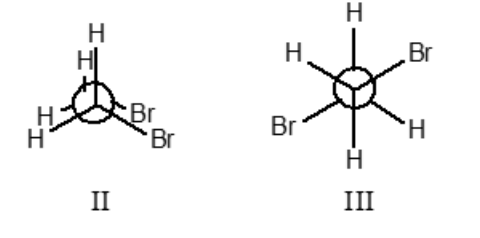
II and III only
-
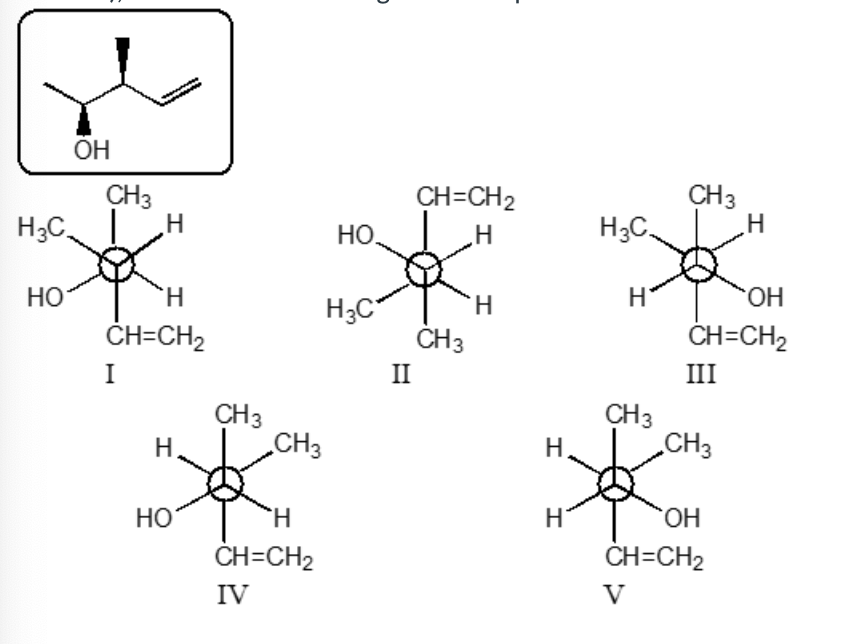
Which staggered Newman projection(s), looking down the C-2—C-3 bond (C-2 in front and C-3 in back), illustrates the following boxed compound?
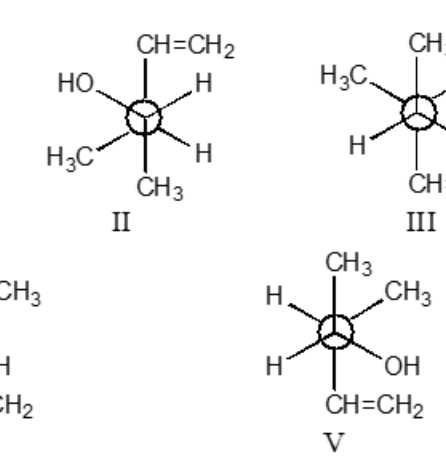
II and V
-
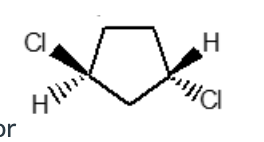
Select the systematic name for this
cis-1,3-Dichlorocyclopentane
trans-1,4-Dichlorocyclopentane
cis-1,2-Dichlorocyclopentane
trans-1,3-Dichlorocyclopentane
1,1-Dichlorocyclopentane
trans-1,3-Dichlorocyclopentane
-
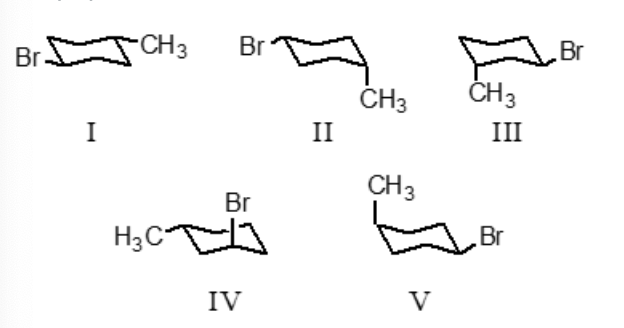
Which conformation represents the most stable conformation of trans-1-bromo-4-methylcyclohexane?
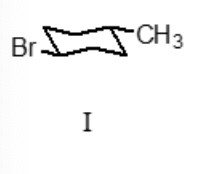
I
-
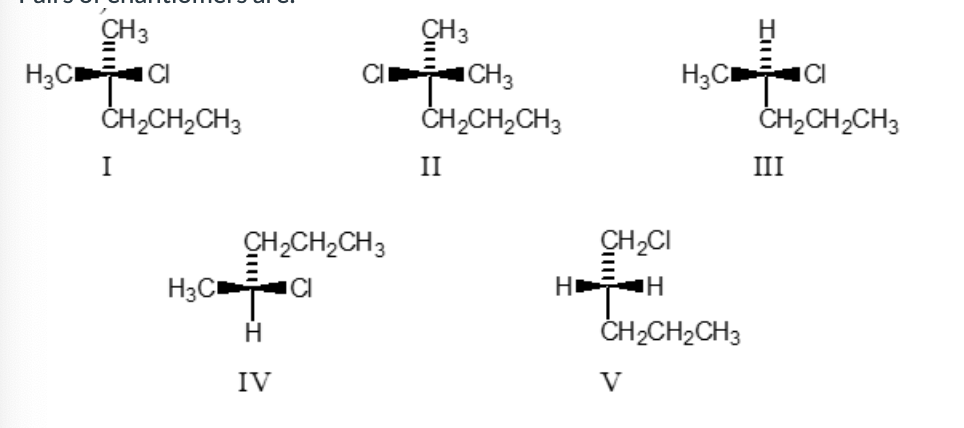
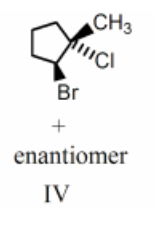
Pairs of enantiomers are
III, IV
-
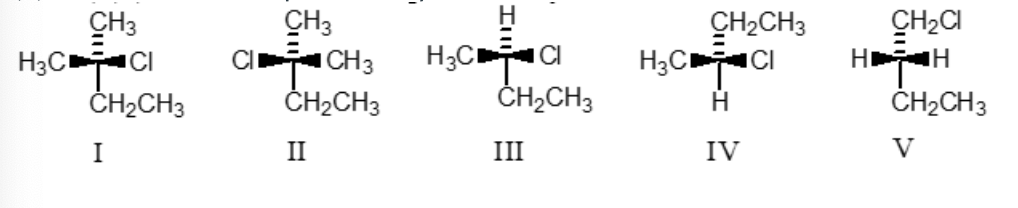
(R)-2-Chlorobutane is represented by:
III
-
Which compound would show optical activity?
cis-1,4-Dimethylcyclohexane
trans-1,4-Dimethylcyclohexane
cis-1,4-Dimethylcycloheptane
trans-1,4-Dimethylcycloheptane
More than one of these choices
trans-1,4-Dimethylcycloheptane
-

75% (R), 25% (S)
25% (R), 75% (S)
50% (R), 50% (S)
67% (R), 33% (S)
33% (R), 67% (S)
25% (R), 75% (S)
-
CH3CHBrCH2CHClCH3 is the generalized representation of what number of stereoisomers?
4
-
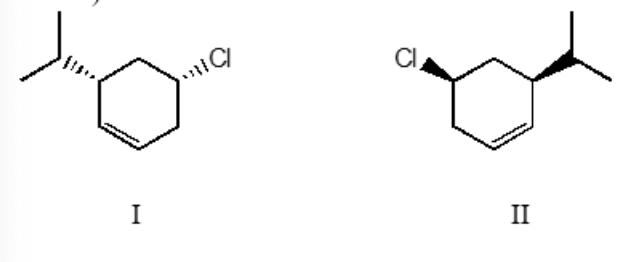
I and II are
constitutional isomers
enantiomers
identical
diastereomers
not isomeric
identical
-
Select the hybridized atomic orbital.


