What are the functions of bone? (5)
Support and movement: Acts as an attachment site for muscles.
Protection: Protects internal organs.
Bone marrow housing: Provides a home for bone marrow.
Mineral reservoir: Acts as a storage site for minerals.
Endocrine function: Produces some 'non-classical' hormones.
What are the two types of bone structure? (2)
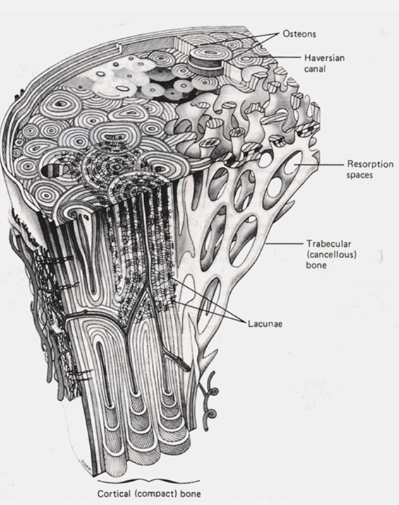
Cortical (compact) bone: Dense, outer layer of bone.
Trabecular (spongy, cancellous) bone: Lighter, inner layer with a honeycomb-like structure.
What are the two types of bone structure? (2)
Cortical (compact) bone: Dense, outer layer of bone.
Trabecular (spongy, cancellous) bone: Lighter, inner layer with a honeycomb-like structure.
What is the composition of bone? (3)
Protein: Organic osteoid matrix (25%)
Mineral: (75%)
Cells: Various bone cells (e.g., osteoblasts, osteocytes, osteoclasts).
What is the organic (osteoid) protein matrix of bone and its function? (2)

Mainly type 1 collagen
Gives flexibility and tensile strength
What is the bone mineral composition and its function? (3)

Hydroxyapatite
Calcium and phosphate (Ca₁₀(PO₄)₆(OH)₂)
Rigid, brittle: provides high compressive strength
What are the main types of bone cells and their roles? (5)
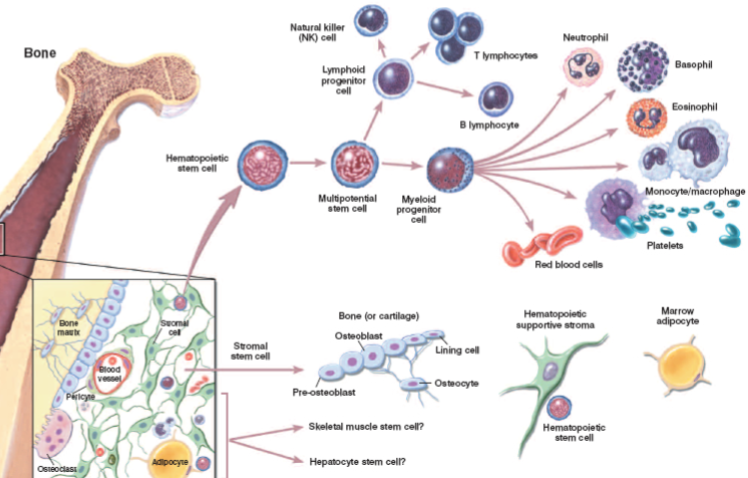
Osteoblasts: Responsible for bone formation.
Osteocytes: Mature bone cells that maintain the bone matrix.
Osteoclasts: Cells responsible for bone resorption (breakdown).
Bone marrow cells: Involved in blood cell production.
Mesenchymal (stromal) stem cells: Can differentiate into various cell types, including osteoblasts.
Haematopoietic stem cells: Give rise to all blood cells.
Picture demonstrating Bone remodelling:
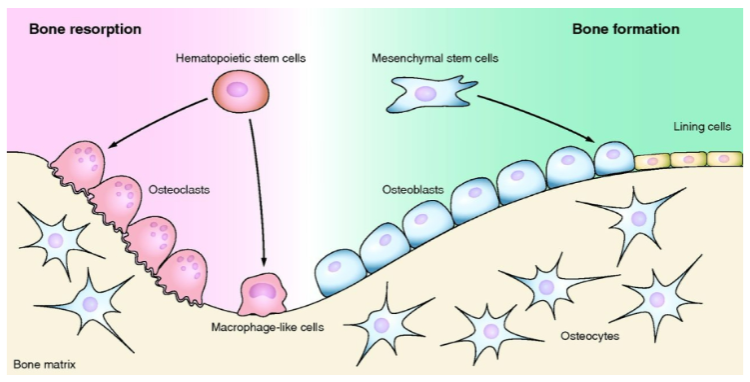
How does bone contribute to metabolic homeostasis? (4)
Bone turnover helps maintain serum calcium and phosphate levels.
Parathyroid hormone (PTH): Increases bone resorption to raise calcium levels.
Vitamin D (1,25-dihydroxy D3): Enhances calcium absorption and bone mineralization.
Calcitonin: Lowers blood calcium by inhibiting osteoclast activity.
FGF-23: Regulates phosphate homeostasis by reducing renal phosphate reabsorption and inhibiting vitamin D synthesis.
What is the daily calcium turnover and recommended intake? (3)
![<p><strong>Daily calcium intake</strong>: 1000-1200 mg (25-30 mmol).</p><p><strong>Extracellular plasma calcium concentration</strong>: 2.2-2.6 mmol/L.</p><p><strong>Calcium distribution</strong>: About half of calcium is free [Ca²⁺] (physiologically active), and the other half is protein-bound (mainly to albumin).</p>](/flashcards/cardimage2/3eda33f/782/8782180_back.png)
Daily calcium intake: 1000-1200 mg (25-30 mmol).
Extracellular plasma calcium concentration: 2.2-2.6 mmol/L.
Calcium distribution: About half of calcium is free [Ca²⁺] (physiologically active), and the other half is protein-bound (mainly to albumin).
What are the key hormones with skeletal effects? (6)
Oestrogen
Androgens
Cortisol
Parathyroid hormone (PTH)
Vitamin D (calcitriol)
Calcitonin
What hormone is secreted from the skeleton? (1)
FGF-23 (fibroblast growth factor 23)
Where is parathyroid hormone (PTH) synthesized? (1)
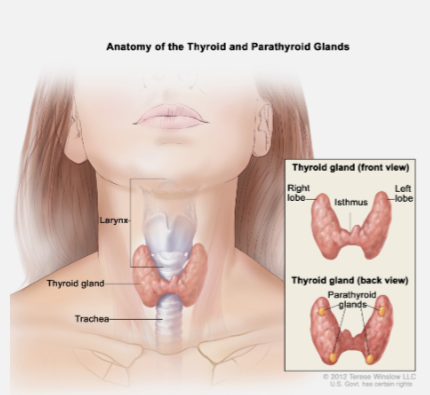
Parathyroid chief cells
What is the function of parathyroid hormone (PTH)? (1)
![<p>Increases serum calcium levels [Ca2+]</p>](/flashcards/cardimage2/5be5f89e/787/8787038_back.png)
Increases serum calcium levels [Ca2+]
What are the characteristics of parathyroid hormone (PTH) secretion? (2)
Secreted as an 84-amino acid (AA) polypeptide
Has a short half-life in circulation (<5 minutes)
What is the role of parathyroid hormone (PTH) in the body? (1)
It is the major hormone involved in calcium homeostasis.
What is the normal plasma calcium concentration range? (2)
Total calcium: 2.2–2.6 mM
Ionized calcium (Ca2+): Approximately half of total calcium
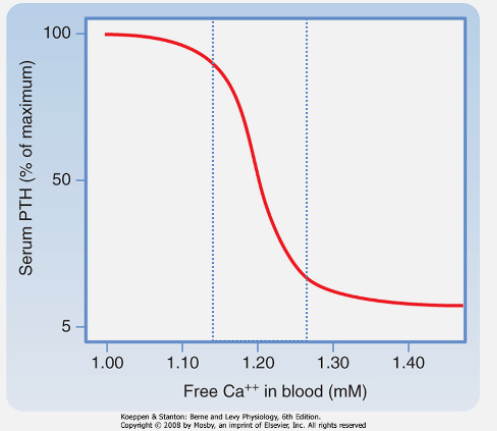
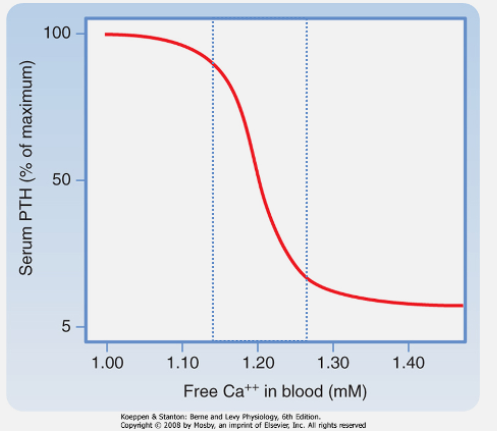
How do parathyroid chief cells sense calcium levels? (1)
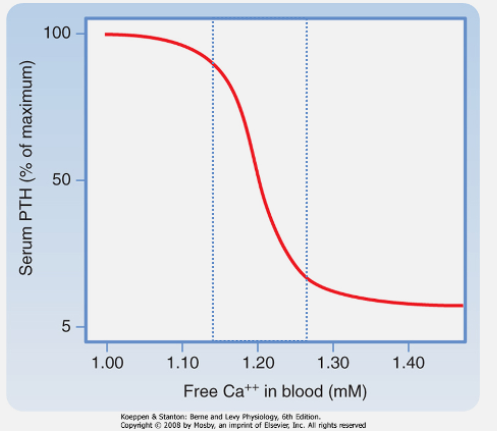
Free (ionized) Ca2+ is sensed by G-protein-coupled receptors (GPCR) on chief cells.
What effect does calcium binding to the calcium-sensing receptor (CaSR) of the PTH glands have on PTH release? (1)
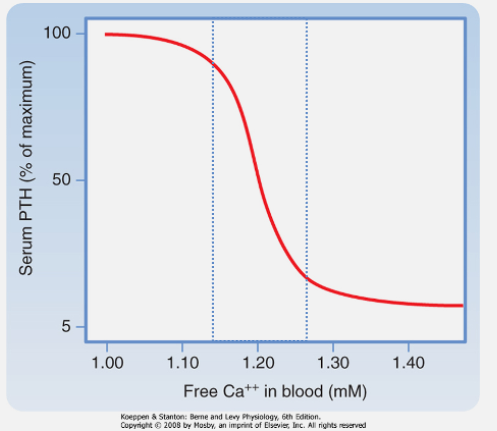
Calcium binding suppresses PTH release
What is calcitriol, and why is it sometimes misclassified? (2)
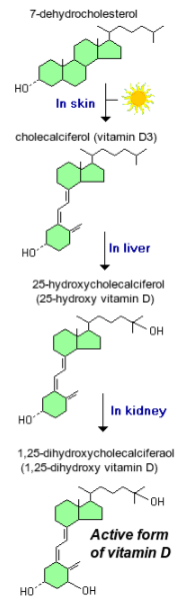
Calcitriol is a steroid hormone, not a true vitamin.
It is often called the "sunshine vitamin" because it is synthesized in the skin in response to UV exposure.
How is vitamin D activated in the body? (3)
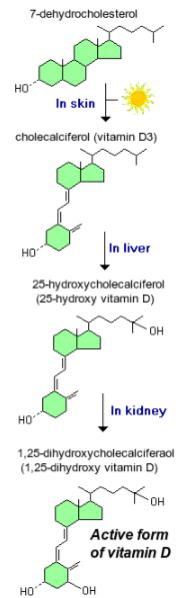
25-hydroxylation occurs in the liver to form 25OH D₃, the major circulating metabolite.
1α-hydroxylation of 25OH D₃ (25-hydroxyvitamin D₃) occurs in the kidney to produce 1,25(OH)₂ D₃ (calcitriol), the active hormone.
What is the main function of calcitriol in calcium homeostasis? (1)
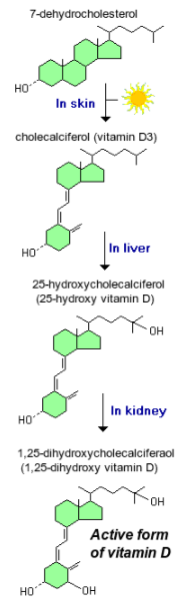
It is needed for calcium absorption from the gut.
What regulates the secretion of PTH from parathyroid chief cells? (1)
PTH secretion increases as plasma [Ca2+] decreases.
What are the actions of parathyroid hormone (PTH)? (4)
Promotes release of calcium from bone.
Increases renal calcium reabsorption.
Increases renal phosphate excretion.
Upregulates 1α-hydroxylase activity in the kidney.
What regulates the activation of calcitriol (Vitamin D)? (2)
Controlled by 1α-hydroxylase activity in the kidney.
Increased by PTH and low phosphate levels.
What are the actions of calcitriol (Vitamin D)? (4)
Increases absorption of calcium and phosphate from the gastrointestinal (GI) tract.
Inhibits PTH secretion by reducing PTH transcription.
Has complex effects on bone, generally acting in synergy with PTH.
Little calcium or phosphate absorption occurs in its absence.
Where is most of the body's calcium stored? (1)
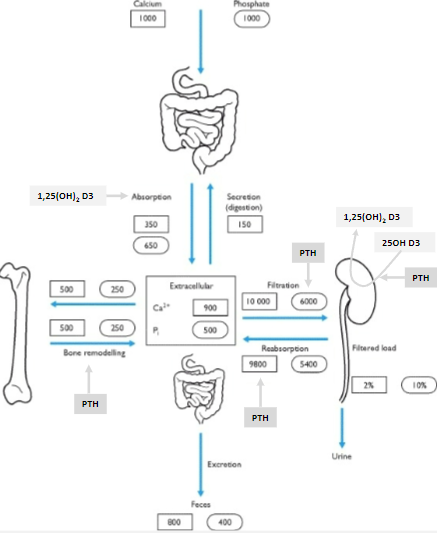
99% of the body's calcium is in the bone.
How is the remaining 1% of body calcium distributed? (2)
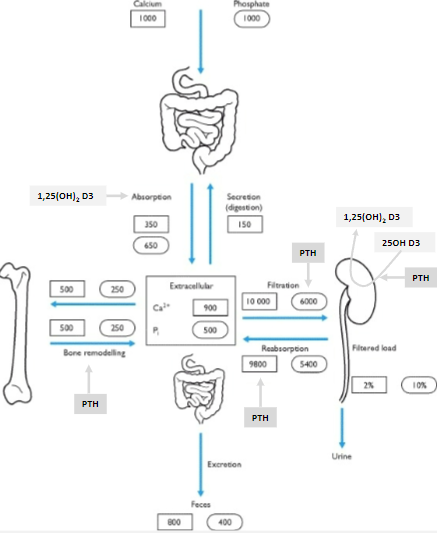
The majority is intracellular.
Less than 0.1% is extracellular.
What maintains calcium balance in the body? (1)
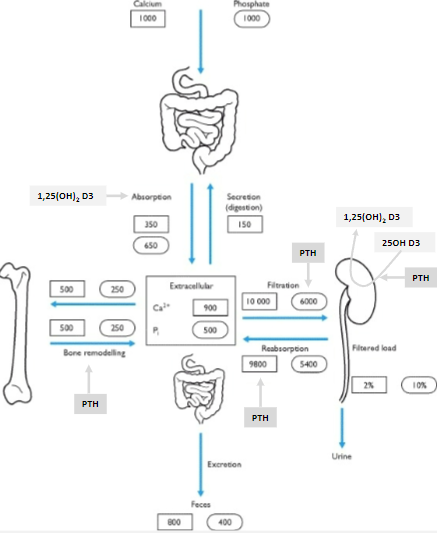
Hormonal control of the tiny extracellular calcium fraction (<0.1%) maintains calcium balance.
Where are the PTH receptors located in bone? (2)
PTH receptors are located on osteoblasts and osteocytes, not on osteoclasts.
Osteoblasts respond to PTH by producing signaling molecules like RANKL, which stimulate osteoclast activity for bone resorption.
How does PTH activate osteoclasts? (1)
PTH activates osteoclasts via RANKL.
What is the effect of PTH on bone remodeling? (1)
PTH promotes bone remodeling.
How does the effect of PTH on bone depend on its concentration dynamics? (2)

Intermittent low doses of PTH are anabolic, promoting bone formation.
Persistent high concentrations of PTH are catabolic, leading to excess resorption over formation and bone loss.
How do osteocytes function as endocrine cells? (2)
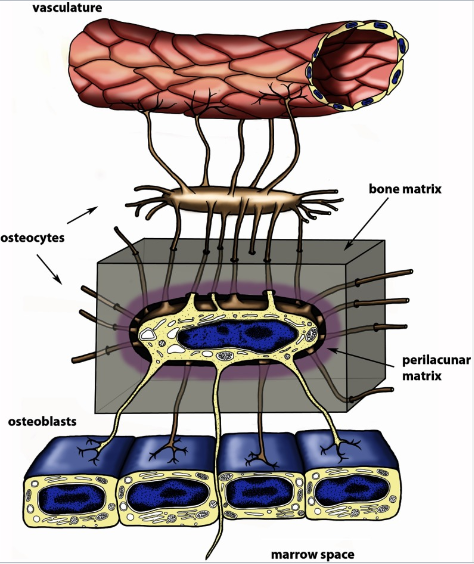
The lacunocanalicular network allows communication between osteocytes.
It also facilitates communication from osteocytes to surface cells and the systemic circulation.
What is the function of the lacunocanalicular network in bone? (2)
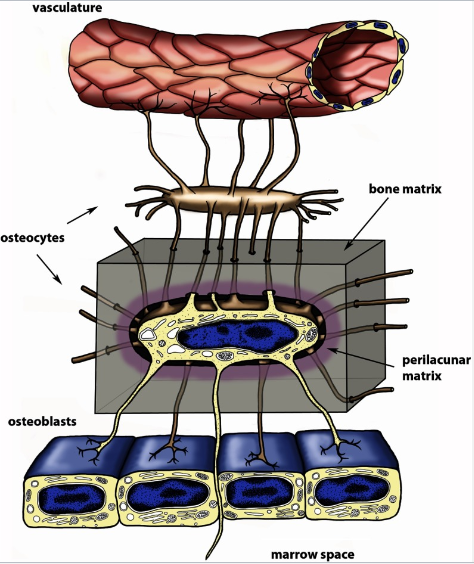
It facilitates communication between osteocytes.
It enables communication between the systemic circulation and bone.
When was FGF-23 discovered? (1)
FGF-23 was discovered in 2000.
What condition is associated with FGF-23 and its mutations? (2)
FGF-23 mutations are linked to hypophosphatemic rickets, a rare phosphate-wasting condition.
This condition leads to bone mineralization defects, such as osteomalacia.
What discovery did the consortium (group of researchers) investigating autosomal-dominant hypophosphatemic rickets (ADHR) make? (1)
The consortium traced a mutation in a gene that turned out to be FGF-23.
What is the central role of FGF-23? (1)
FGF-23 plays a central role in phosphate homeostasis.
Where is FGF-23 expressed and secreted? (1)
FGF-23 is expressed and secreted by osteocytes.
What is the action of FGF-23 on phosphate (Pi) excretion? (1)
FGF-23 increases renal phosphate excretion by reducing Na-Pi reabsorption from the proximal tubule.
What factors increase FGF-23 secretion? (2)
FGF-23 secretion is increased by calcitriol and phosphate (Pi).
How does FGF-23 affect calcitriol synthesis? (1)
FGF-23 inhibits calcitriol synthesis.
What is osteoporosis? (1)
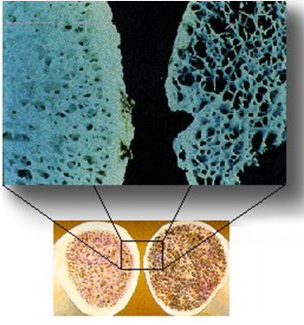
Osteoporosis is the loss of bone mass, including both mineral and organic matrix.
What are the main causes of osteoporosis? (6)
Endocrine disorders
Malignancy
Drug-induced factors
Renal disease
Nutritional deficiencies
Age
What is osteomalacia? (1)
Osteomalacia is the loss of bone mineralization, known as rickets in children.
What are the signs and symptoms of osteomalacia? (4)
Permanent deformities in bone growth (rickets)
Diffuse aches and pains
Chronic fatigue
Weak bones
What are the laboratory characteristics of osteomalacia? (3)
Low calcium (Ca) and/or phosphate (Pi) levels
Elevated alkaline phosphatase (ALP)
Parathyroid hormone (PTH) may be elevated
What are the causes of osteomalacia? (3)
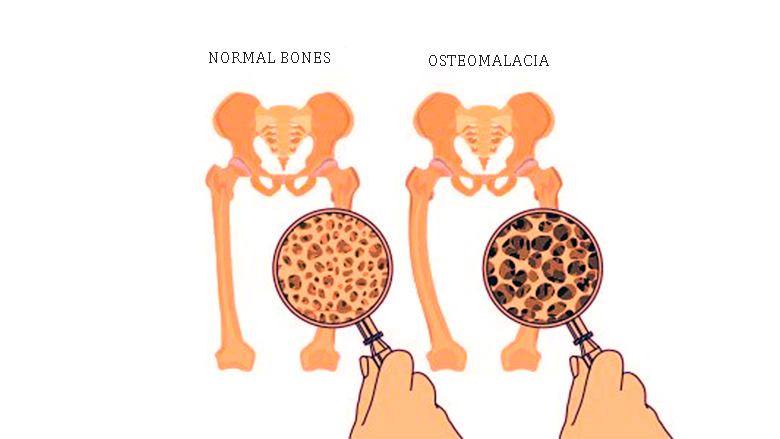
Vitamin D deficiency (most common)
Vitamin D metabolism defects (rare)
Phosphate wasting (rare)
What are the endocrine causes of osteoporosis? (4)
Hypogonadism, notably any cause of estrogen deficiency.
Excess glucocorticoids (endogenous or exogenous).
Hyperparathyroidism.
Hyperthyroidism.
What is the normal range of calcium in the body? (1)
The normal calcium range is 2.2 – 2.6 mM.
What are the clinical features of hypercalcemia? (6)
Depression, fatigue, anorexia, nausea, and vomiting.
Abdominal pain and constipation.
Renal calcification (kidney stones).
Bone pain (referred to as "painful bones, renal stones, abdominal groans, and psychic moans").
Severe hypercalcemia can lead to cardiac arrhythmias and cardiac arrest.
What are the most common causes of hypercalcemia? (2)
In ambulatory patients: primary hyperparathyroidism.
In hospitalized patients: malignancy.
What are less common causes of hypercalcemia? (2)
Hyperthyroidism.
Excessive intake of vitamin D.
What is the most common cause of primary hyperparathyroidism? (1)
Primary hyperparathyroidism is usually due to a benign adenoma in one or more parathyroid glands.
How is primary hyperparathyroidism often detected? (1)
It is often detected on screening, and many patients are asymptomatic (person does not exhibit any noticeable symptoms of a condition or disease).
What percentage of patients with primary hyperparathyroidism present with clinical evidence of bone disease? (1)
Approximately 10% of patients present with clinical evidence of bone disease.
What percentage of patients with primary hyperparathyroidism present with kidney stones? (1)
10-20% of patients present with kidney stones
How is primary hyperparathyroidism treated? (1)
It is resolved by surgical removal of the affected parathyroid gland(s).
What is the common cause of hypercalcemia in advanced malignancy? (1)
Hypercalcemia in malignancy is commonly caused by the secretion of PTH-related peptide by the tumor, which binds and activates the PTH receptor.
What does Bone Mineral Density (BMD) measure? (1)
BMD measures the amount of mineral in bone tissue.
How is Bone Mineral Density (BMD) measured? (1)
It is measured using a scanning process called dual-energy X-ray absorptiometry (DXA or DEXA).
What does low BMD indicate? (1)
Low BMD (Bone Mineral Density) indicates an increased risk of fractures.
What is osteoporosis? (1)
Osteoporosis is severe loss of BMD, leading to thinner, weaker bones and increased fracture risk.
What is osteomalacia? (1)
Osteomalacia is a pathological condition where new bone cannot be mineralized due to vitamin D/Calcium/Phosphate Deficiency.
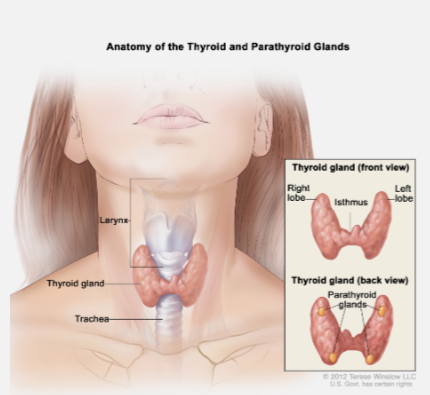
Where are the parathyroid glands located? (1)
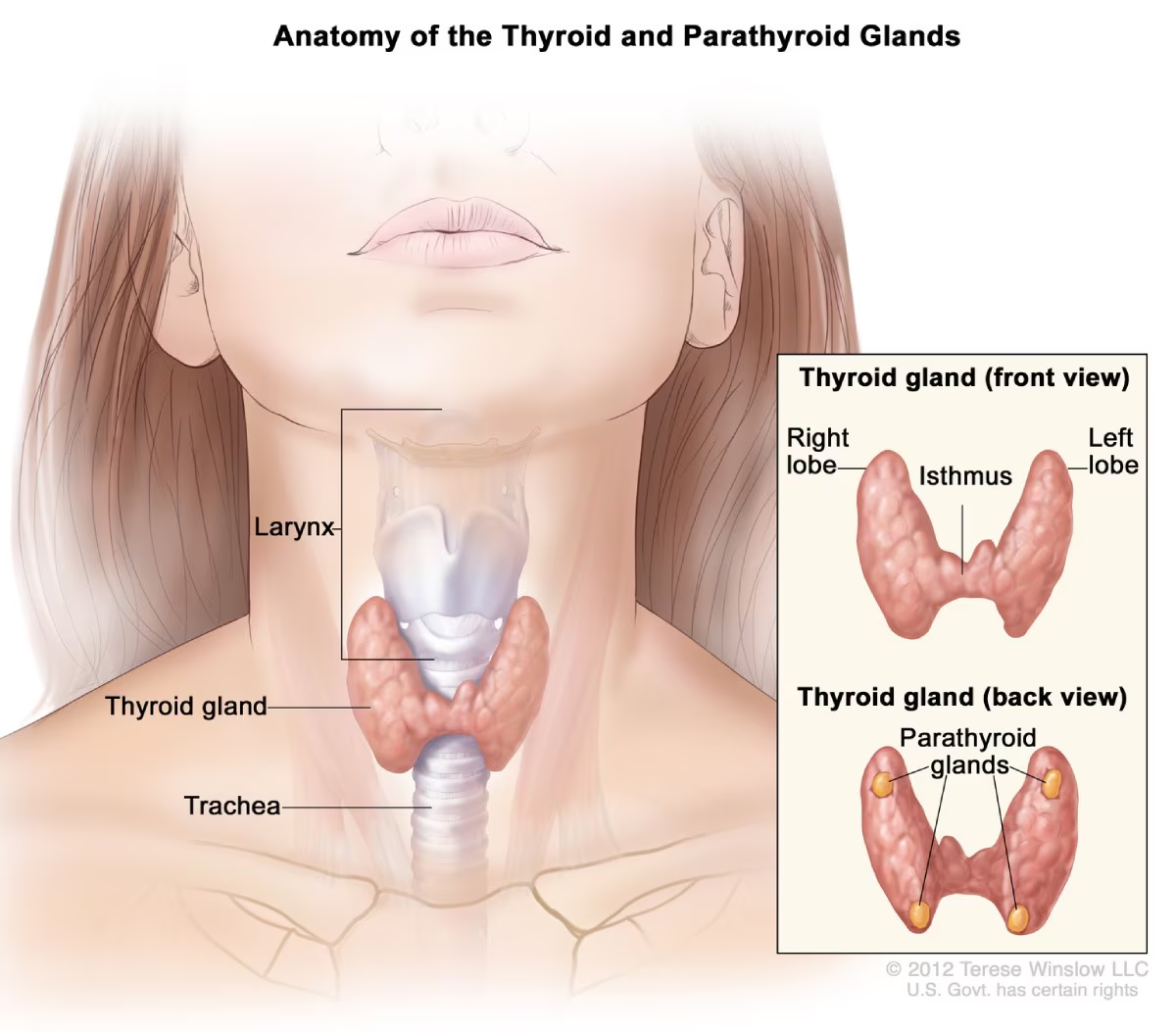
The parathyroid glands are four small glands located on the back of the thyroid.
What does the parathyroid hormone (PTH) do? (1)
PTH maintains plasma calcium within physiological limits.
How does PTH respond to changes in plasma calcium levels? (1)
A decrease in plasma calcium levels increases PTH secretion.
What is the effect of intermittent and continuous PTH treatment on bone mineral density (BMD)? (2)
Intermittent treatment with synthetic PTH has an anabolic effect, increasing bone formation.
Continuous treatment with PTH is catabolic, resulting in the loss of BMD.
What is Vitamin D classified as? (1)
Vitamin D is strictly a hormone, not a vitamin.
How is Vitamin D synthesized and activated? (3)
Vitamin D3 (Cholecalciferol) is synthesized in the skin in the presence of sunlight, but it can also be obtained from the diet.
Vitamin D3 (Cholecalciferol) is biologically inert.
It is converted into 25-hydroxycholecalciferol (25(OH)D3), a prohormone, by the liver.
25-hydroxycholecalciferol (25(OH)D3) is then converted into the active form, 1,25-dihydroxycholecalciferol (1,25(OH)₂D3) (calcitriol), by a hydroxylase enzyme in the kidney.
What is FGF-23 and how does it function? (3)
FGF-23 is a hormone produced by osteocytes in response to high plasma phosphate (PO4) levels.
It acts in the kidney to decrease phosphate reabsorption (prevents hyperphosphatemia).
It also acts on the parathyroid to inhibit PTH release.