What is the "functional genome" and its relevance to genetic diagnosis? (3)
The functional genome refers to understanding how genetic variations affect gene function and contribute to disease.
It involves whole genome sequencing (WGS) and whole exome sequencing (WES) to identify potential disease-causing genetic variants.
Functional validation through in vitro and in vivo methods is needed to confirm whether a variant is pathogenic.
What are the steps in the pipeline to genetic diagnosis? (5)
Identify genetic variants through WES or WGS.
Filter and prioritize candidate genes based on bioinformatics and family co-segregation.
Validate variants with techniques like Sanger sequencing.
Functional assays are needed to test how the variants affect gene/protein function (e.g., immunohistochemistry, cell biology, protein interactions).
In vitro (e.g., cell culture, CRISPR) and in vivo (e.g., mouse, zebrafish) models are developed to study the effects on phenotype, protein behavior, and tissue development.
What is the role of bioinformatics in WES and WGS? (3)
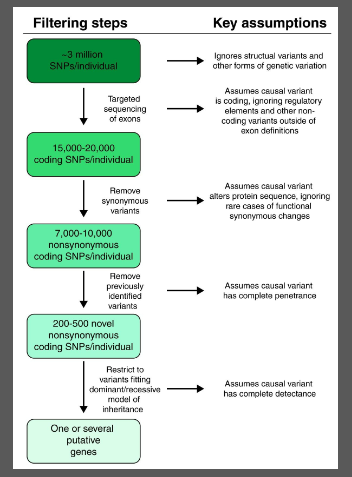
WES and WGS generate a large amount of genetic data.
Bioinformatics tools are used to filter through 15-20,000 coding SNPs and prioritize potential disease-causing variants.
Candidate gene filtering narrows down the focus to a smaller set of genes for validation and further testing.
Why is functional validation important in genetic diagnosis? (2)
Filtered variants from WES/WGS do not prove causality.
Functional evidence is essential to confirm whether a genetic variant is indeed pathogenic and how it affects the gene, protein, and cell function.
What are in vitro techniques used to study patient variants? (4)
In vitro techniques involve removing cells from animals or humans and growing them in controlled conditions.
Cell culture provides a rapid and reproducible model for studying normal cell physiology and biochemistry.
Gene knockdown can be achieved using SiRNA or ShRNA to silence specific genes and observe effects.
These methods offer an alternative to animal models and help reduce ethical concerns.
Why might a gene of interest (GOI) not be expressed in blood or tissue biopsies? (2)
Some genes are tissue-specific and may not be expressed in blood or easily accessible tissues.
For example, MYL1, a gene linked to congenital muscular dystrophy, is expressed in fast-twitch muscle, not blood, and may not show up in biopsies.
How can gene mutations be detected in patient samples? (3)
Western blot analysis can detect protein levels and confirm if a mutation causes loss of function.
For example, a mutation in MYL1 in muscular dystrophy patients results in reduced MYL1 protein.
This can be confirmed by comparing protein expression in patient samples to healthy controls.
What is gene knockdown in cell culture and how is it achieved? (4)
Gene knockdown involves reducing the expression of a specific gene to study its function.
SiRNA (short interfering RNA) and ShRNA (short hairpin RNA) are used for gene silencing.
SiRNA is chemically synthesized, while ShRNA is plasmid-based and works through RNA-induced silencing complex (RISC) to degrade mRNA.
Both methods can be used to silence genes and observe the resulting phenotypic changes in cell culture.
What is the role of SiRNA and ShRNA in gene silencing? (3)
SiRNA is synthesized to directly degrade complementary mRNA and silence genes.
ShRNA is a plasmid-based method that mimics natural microRNA for gene silencing.
Both methods interfere with gene expression by leading to mRNA degradation via RISC.
How can the localization of a gene of interest (GOI) encoded protein be determined? (3)
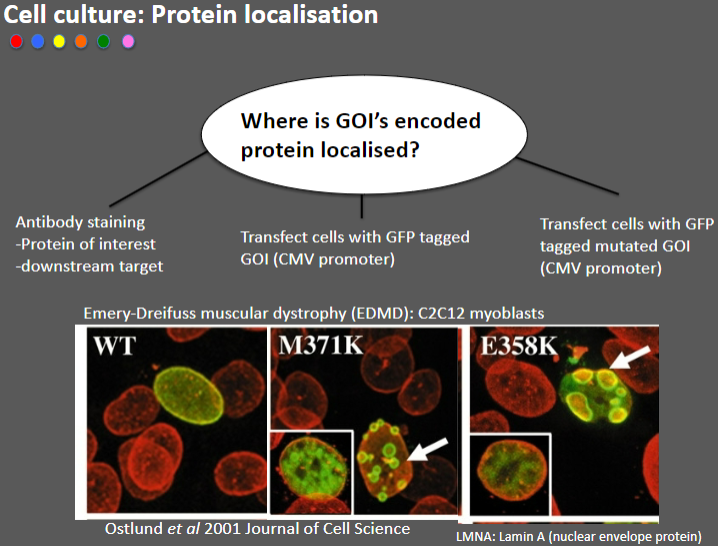
Antibody staining: Used to detect the protein of interest or its downstream target by binding specific antibodies to the protein.
Transfect cells with GFP-tagged GOI: This method attaches Green Fluorescent Protein (GFP) to the GOI, allowing visualization of its location in the cell using fluorescence microscopy.
Transfect cells with GFP-tagged mutated GOI: This allows comparison of the localization of the wild-type and mutated versions of the protein.
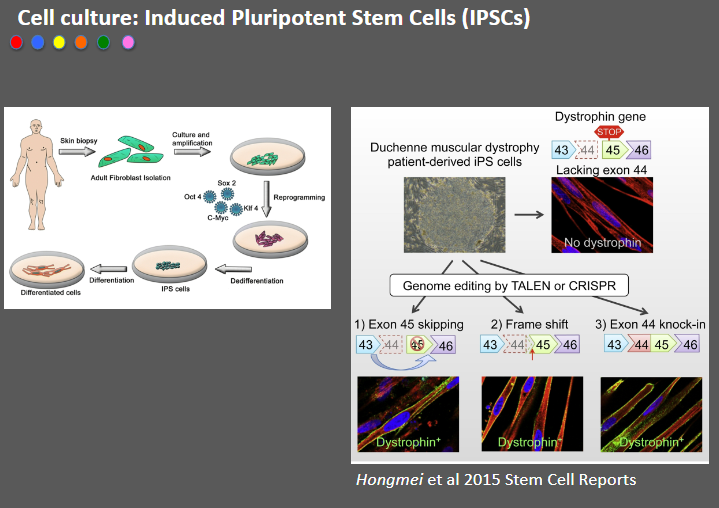
Picture demonstrating Cell culture: Induced Pluripotent Stem Cells (IPSCs):
Why is cell culture not enough for studying gene expression and function in developmental phenotypes? (3)
Cells behave differently in a petri dish/flask: Cultured cells exist in a 2D environment, which doesn't fully represent their behavior in a living organism.
Lack of information on gene expression and function: In vitro models do not simulate the complex interactions and conditions that affect gene function, particularly in developmental contexts.
No simulation of organism conditions: Cells in culture lack signals from other tissues, which are critical for understanding the full developmental phenotype of genes.
Why are animals used in research despite ethical concerns? (3)
Cells behave differently in vivo: In vitro models do not replicate the full complexity of organisms, making animal studies essential for accurate biological insights.
Animal research has contributed significantly to medicines: Many modern medicines are derived from animal research, with animals playing a critical role in their development.
Nobel Prize contributions: Animal research has contributed to 70% of Nobel Prizes in the field of medicine, demonstrating its importance in scientific progress.
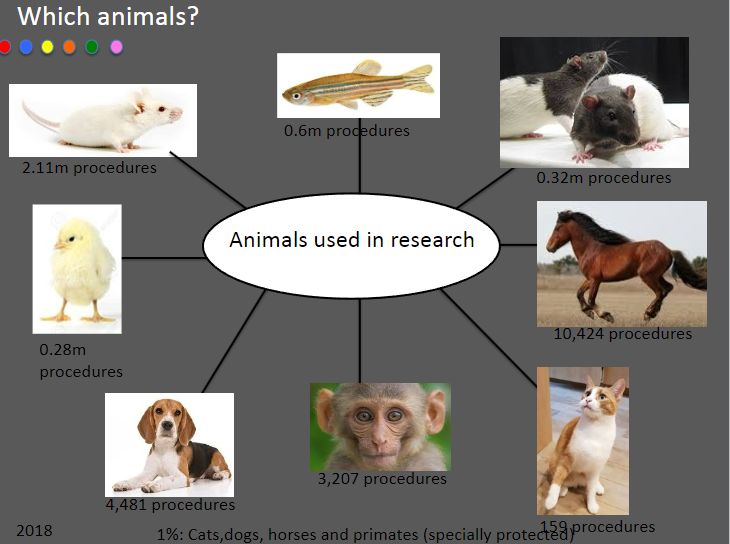
Picture demonstrating animals used in research:
Why are mice used as a model for human genetic disease research? (5)
Accelerated lifespan: Mice age quickly, with 1 year equivalent to 30 human years, allowing for faster study of disease progression.
Long history in biomedical research: Mice have been used for over 100 years, providing a wealth of data and established methodologies.
Small size and rapid reproduction: Mice are easy to handle, breed quickly, and require less space for housing.
Genetic similarity to humans: Mice are mammals, making them genetically similar to humans, ideal for studying genetic diseases.
Ethical advantages: Using mice is more ethical than using larger animals or non-human primates in research.
How is a mutant mouse made? (4)
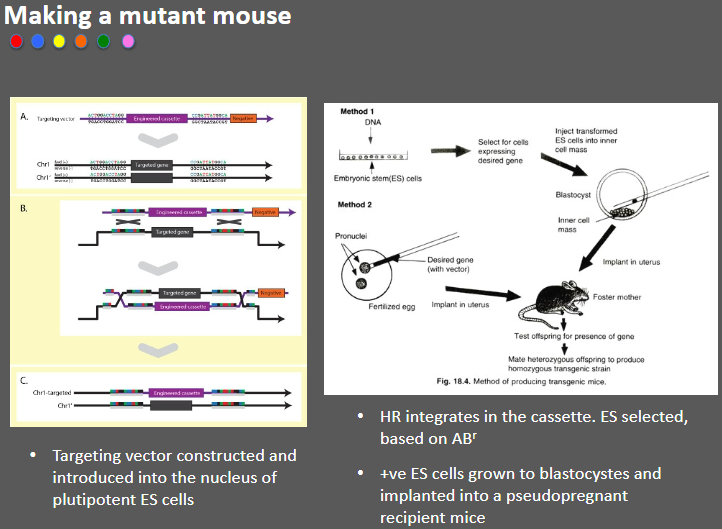
Targeting vector: A targeting vector is constructed and introduced into the nucleus of pluripotent embryonic stem (ES) cells.
Homologous recombination (HR): The vector integrates into the genome through HR, placing the desired genetic modification in the cassette.
Selection of positive ES cells: ES cells are selected based on antibiotic resistance (ABr) markers.
Implantation: Positive ES cells are grown into blastocysts and implanted into pseudopregnant recipient mice.
What are the limitations of Tissue-specific knockout (KO) mice (3)
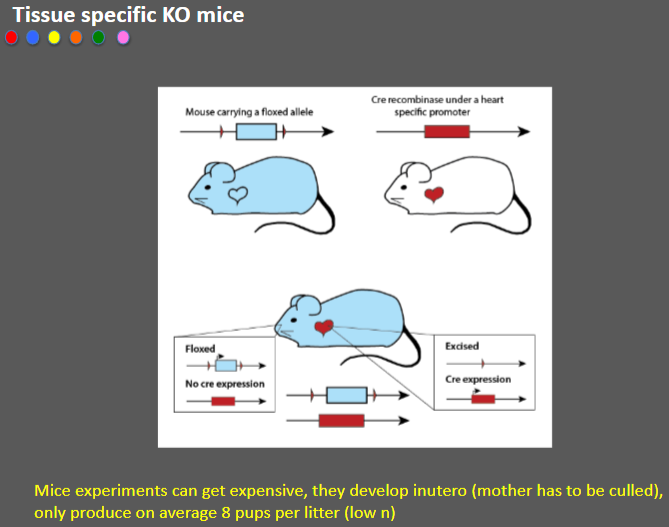
Costly experiments: Mice experiments can be expensive due to the need for specialized resources.
In utero development: Mice develop in utero, requiring the culling of the mother, which raises ethical concerns.
Low litter size: Mice typically produce only around 8 pups per litter, leading to a low sample size (n).
Picture demonstrating Zebrafish: a vertebrate model for human genetic disease:
Outline Zebrafish Gene Knockdown (4)
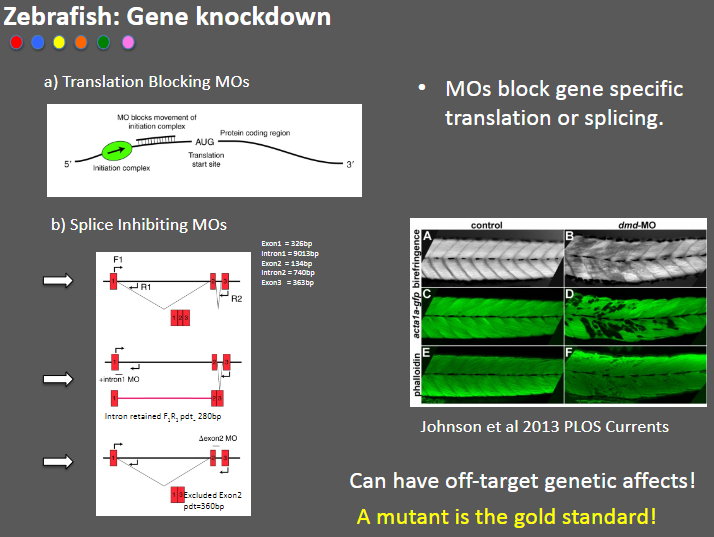
Translation Blocking MOs: Morpholino oligonucleotides (MOs) that block gene-specific translation.
Splice Inhibiting MOs: MOs that inhibit splicing by targeting specific exons (e.g., Exon1 = 326bp).
Off-target effects: MOs can cause unintended genetic changes, leading to off-target effects.
Mutant as gold standard: A true mutant model is considered the gold standard for studying gene function.
What is CRISPR and how does it work? (8)
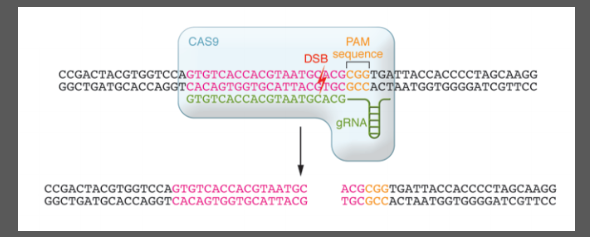
CRISPR is a bacterial immune system with clustered short palindromic repeats.
Cas9 (CRISPR-associated protein 9) is an endonuclease that cuts DNA.
The Protospacer is the target sequence of guide RNA.
The PAM (Protospacer Adjacent Motif) is a short sequence needed for Cas9 to bind and cut DNA.
Cas9 binds to the guide RNA and PAM, creating a Double-Strand Break (DSB) 3 base pairs upstream of PAM.
Mutations are introduced through NHEJ (Non-Homologous End Joining) or HDR (Homology-Directed Repair).
RNA rescue experiments: How do they prove pathogenesis in autosomal recessive cerebellar ataxias (ARCAs)? (4)
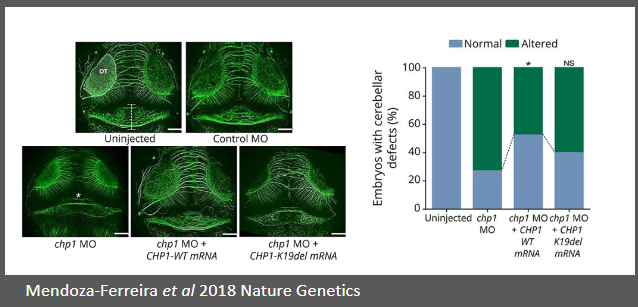
Autosomal recessive cerebellar ataxias (ARCAs) are neurodegenerative disorders.
WES (Whole Exome Sequencing) identifies mutations, such as the one in CHP1.
Mutation in CHP1 causes dysfunction in cerebellar function, leading to ataxia.
RNA rescue experiments introduce functional RNA to rescue or confirm the pathogenic effect of mutations.
What is the role of WES and WGS in identifying candidate causative genes and their follow-up? (5)
WES (Whole Exome Sequencing) and WGS (Whole Genome Sequencing) identify candidate causative genes.
Functional follow-up experiments are required to prove pathogenesis and establish a genetic diagnosis.
In vitro assays are quick, cheap, and have limited ethical concerns but may not reflect organism behavior.
Animal models are essential for understanding disease aetiology but are costly and have strict regulations.
A combination of in vitro and in vivo data provides strong evidence for functional validation of gene variants.