Alanine, Structure
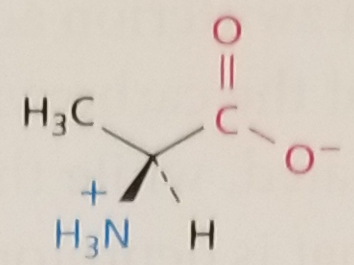
Alanine, Side Chain
Hydrophobic
Alanine, 3-Letter Abbreviation
Ala
Alanine, 1-Letter Abbreviation
A
Arginine, Structure
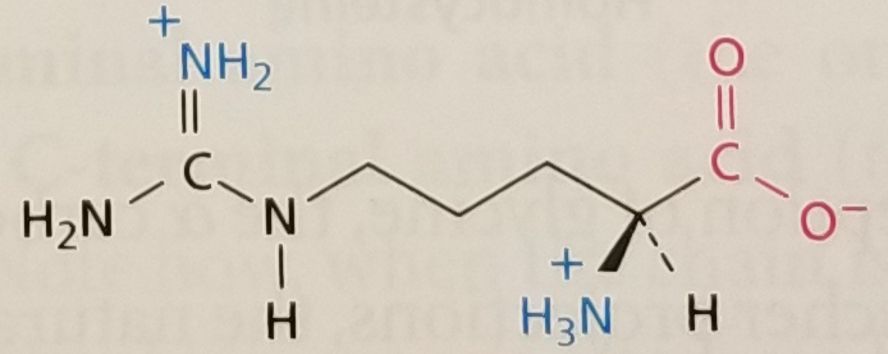
Arginine, Side Chain
Amphipathic, Positively Charged, pKa ~ 12.5
Arginine, 3-Letter Abbreviation
Arg
Arginine, 1-Letter Abbreviation
R
Asparagine, Structure
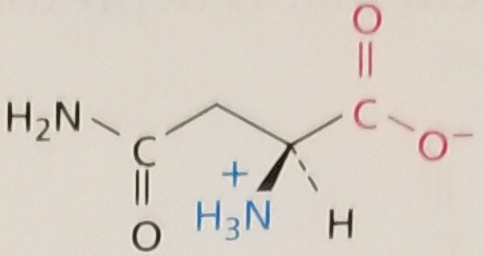
Asparagine, Side Chain
Hydrophilic
Asparagine, 3-Letter Abbreviation
Arn
Asparagine, 1-Letter Abbreviation
N
Aspartic Acid/Aspartate, Structure
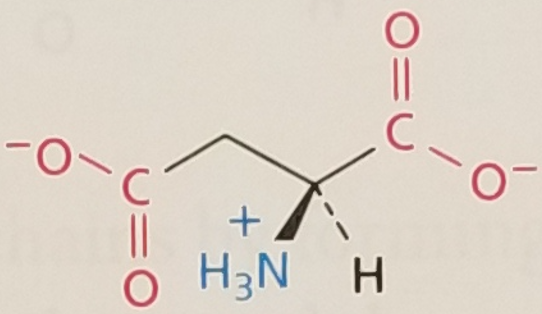
Aspartic Acid/Aspartate, Side Chain
Hydrophilic, Negatively Charged, pKa ~ 3.5
Aspartic Acid/Aspartate, 3-Letter Abbreviation
Asp
Aspartic Acid/Aspartate, 1-Letter Abbreviation
D
Cysteine, Structure
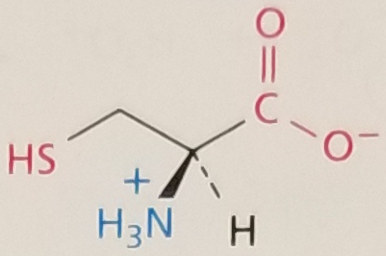
Cysteine, Side Chain
Hydrophobic, Polar Uncharged, pKa ~ 8.5
Cysteine, 3-Letter Abbreviation
Cys
Cysteine, 1-Letter Abbreviation
C
Glutamic Acid/Glutamate, Structure
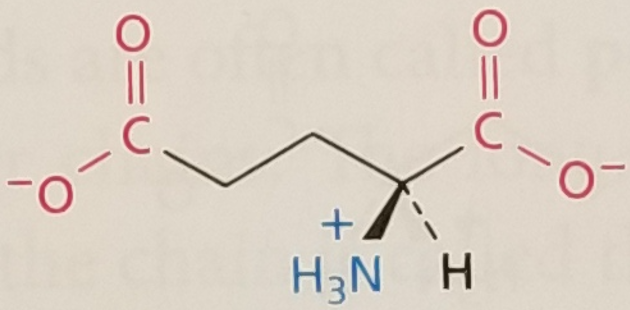
Glutamic Acid/Glutamate, Side Chain
Hydrophilic, Negatively Charged, pKa ~ 4
Glutamic Acid/Glutamate, 3-Letter Abbreviation
Glu
Glutamic Acid/Glutamate, 1-Letter Abbreviation
E
Glutamine, Structure
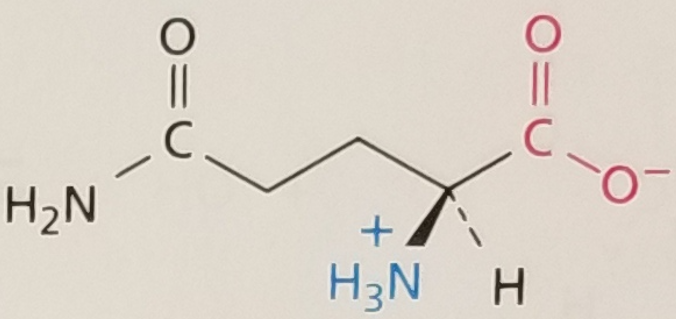
Glutamine, Side Chain
Hydrophilic
Glutamine, 3-Letter Abbreviation
Gln
Glutamine, 1-Letter Abbreviation
Q
Glycine, Structure
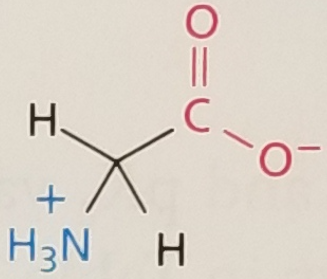
Glycine, Side Chain
Hydrophobic
Glycine, 3-Letter Abbreviation
Gly
Glycine, 1-Letter Abbreviation
G
Histidine, Structure
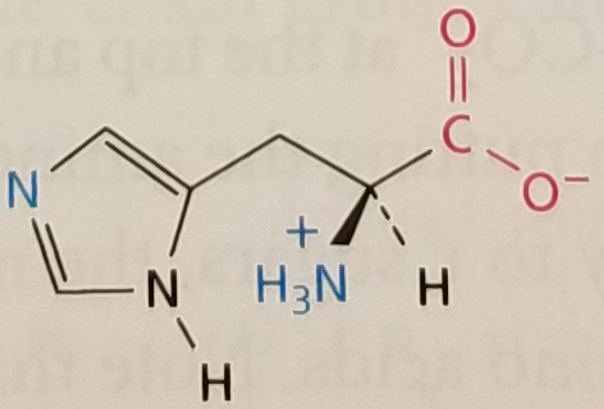
Histidine, Side Chain
Hydrophilic, Positively Charged (classically, it's a bit more complicated than that), pKa ~ 6
Histidine, 3-Letter Abbreviation
His
Histidine, 1-Letter Abbreviation
H
Isoleucine, Structure
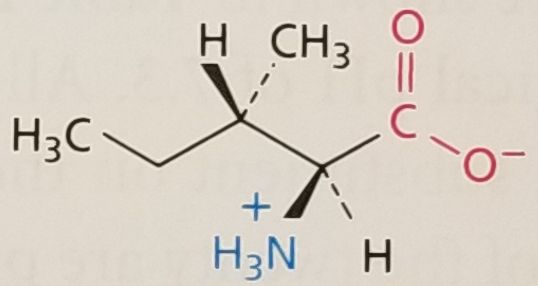
Isoleucine, Side Chain
Hydrophobic
Isoleucine, 3-Letter Abbreviation
Ile
Isoleucine, 1-Letter Abbreviation
I
Leucine, Structure
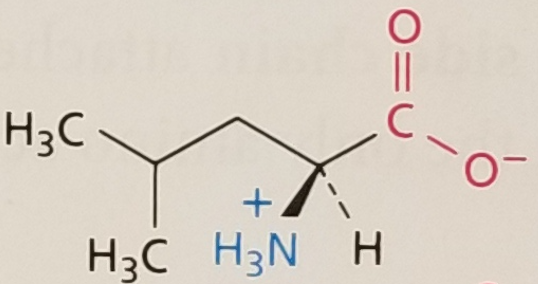
Leucine, Side Chain
Hydrophobic
Leucine, 3-Letter Abbreviation
Leu
Leucine, 1-Letter Abbreviation
L
Lysine, Structure
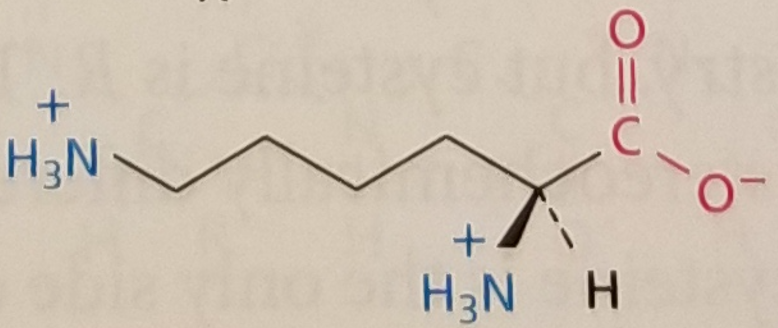
Lysine, Side Chain
Amphipathic, Positively Charged, pKa ~10.5
Lysine, 3-Letter Abbreviation
Lys
Lysine, 1-Letter Abbreviation
K
Methionine, Structure
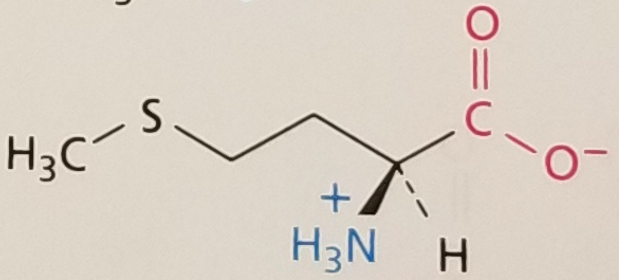
Methionine, Side Chain
Hydrophobic
Methionine, 3-Letter Abbreviation
Met
Methionine, 1-Letter Abbreviation
M
Phenylalanine, Structure
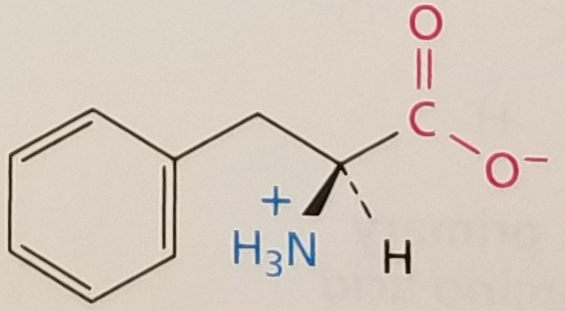
Phenylalanine, Side Chain
Hydrophobic, aromatic
Phenylalanine, 3-Letter Abbreviation
Phe
Phenylalanine, 1-Letter Abbreviation
F
Proline, Structure
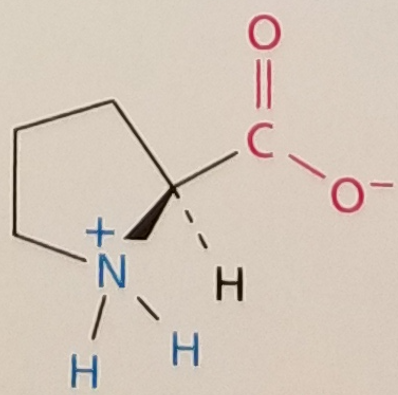
Proline, Side Chain
Hydrophobic
Proline, 3-Letter Abbreviation
Pro
Proline, 1-Letter Abbreviation
P
Serine, Structure
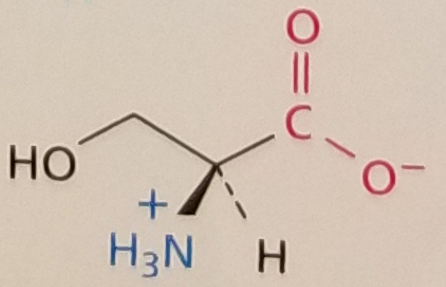
Serine, Side Chain
Hydrophilic
Serine, 3-Letter Abbreviation
Ser
Serine, 1-Letter Abbreviation
S
Threonine, Structure
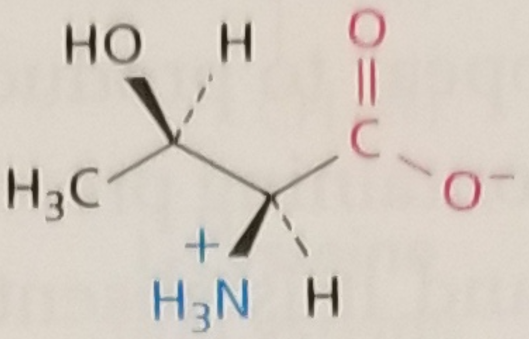
Threonine, Side Chain
Hydrophilic
Threonine, 3-Letter Abbreviation
Thr
Threonine, 1-Letter Abbreviation
T
Tryptophan, Structure
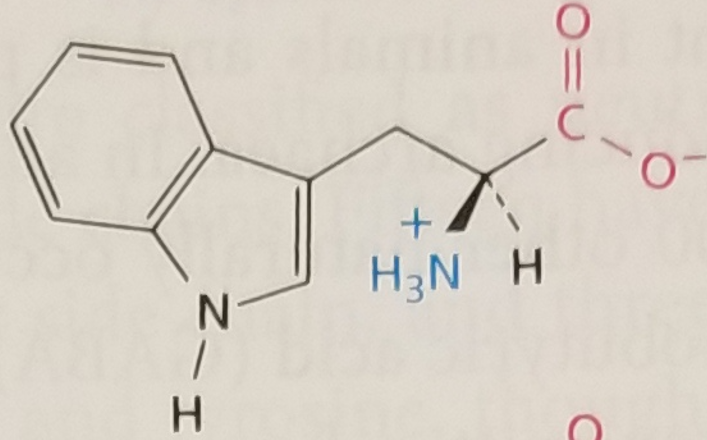
Tryptophan, Side Chain
Amphipathic, aromatic
Tryptophan, 3-Letter Abbreviation
Trp
Tryptophan, 1-Letter Abbreviation
W
Tyrosine, Structure
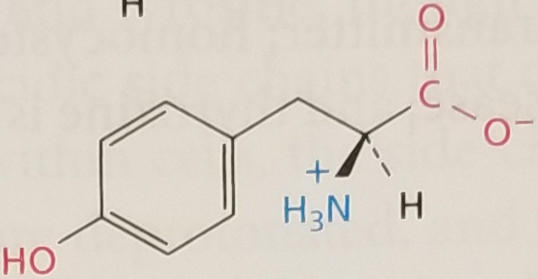
Tyrosine, Side Chain
Amphipathic, aromatic. Polar Uncharged, pKa ~ 10.5
Tyrosine, 3-Letter Abbreviation
Tyr
Tyrosine, 1-Letter Abbreviation
Y
Valine, Structure
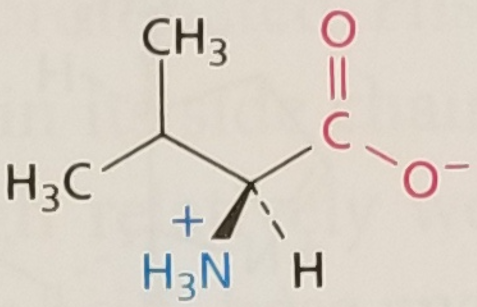
Valine, Side Chain
Hydrophobic
Valine, 3-Letter Abbreviation
Val
Valine, 1-Letter Abbreviation
V
What is the chirality of the isoleucine side chain stereocenter?
S
Which of the amino acids is achiral?
Glycine
All of the common amino acids have an _____ absolute configuration at the alpha-carbon, except _____ which has an _____ stereocenter.
S
cysteine, R
Note: nevertheless, all common amino acids are L-amino acids
What is pKa1 and what is its value for the amino acids?
pKa1 is the pKa for the carboxyl group. It is usually ~2.
What is pKa2 and what is its value for the amino acids?
pKa2 is the pKa for the amino group. It is usually between 9 and 10
What is the chirality of the threonine side chain stereocenter?
R