What are the CNS systems that control behaviour? (3)
Autonomic nervous system
Hypothalamic-pituitary neurohormones
Diffuse monoamine system
What is the function of the sympathetic nervous system? (1)

Body activation
What is the function of the parasympathetic nervous system? (1)

Relaxation and recovery
What are the common principles of the four diffuse modulatory systems of the brain? (4)
Small set of neurons at the core
Arise from the brain stem
One neuron influences many others
Synapses release transmitter molecules into extracellular fluid
What are the four main diffuse modulatory systems of the brain? (4)
Noradrenergic Locus Coeruleus
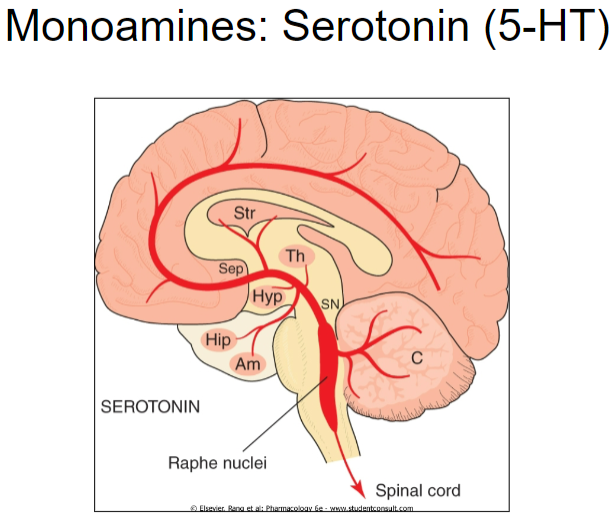
Serotonergic Raphe Nuclei
Dopaminergic Substantia Nigra and Ventral Tegmental Area
Cholinergic Basal Forebrain and Brain Stem Complexes
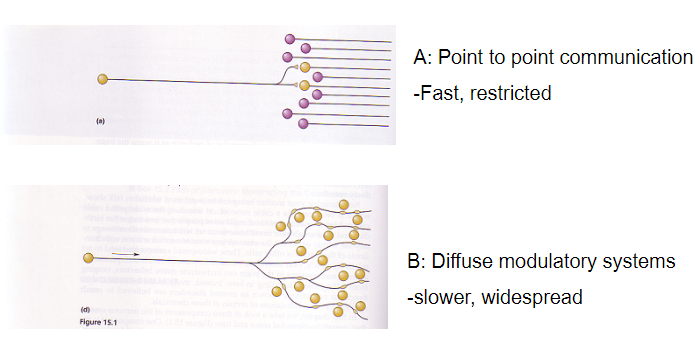
What is the difference between point-to-point communication and diffuse modulatory systems? (2)
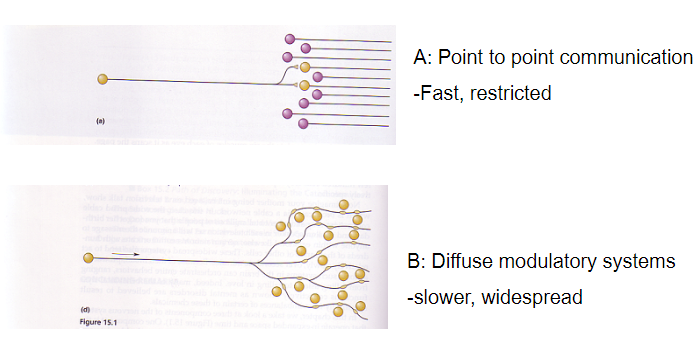
Point-to-point communication: Fast and restricted
Diffuse modulatory systems: Slower and widespread
What are the behavioral effects of diffuse modulatory systems? (5)
Mood
Memory
Reward
Movement
Motivation
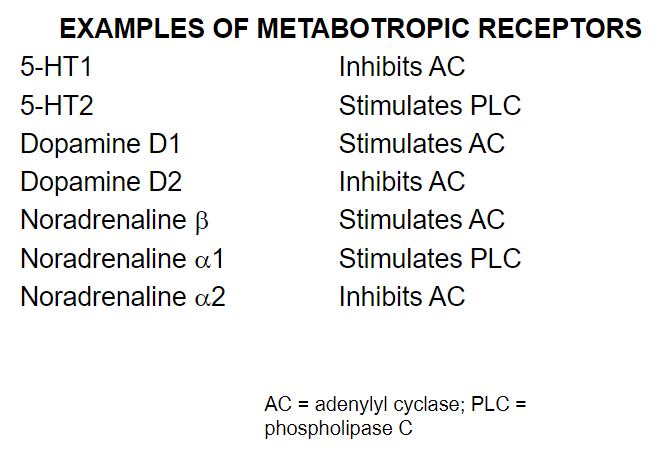
Picture demonstrating examples of metabotropic receptors and what they stimulate:
What are the functions of noradrenaline (NA)? (5)
Arousal
Wakefulness
Exploration and mood (low NA in depression)
Blood pressure
Addiction/gambling
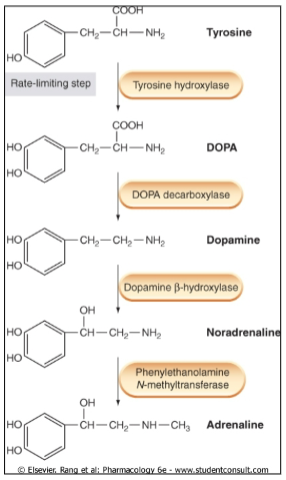
Picture demonstrating the synthesis of catecholamines:
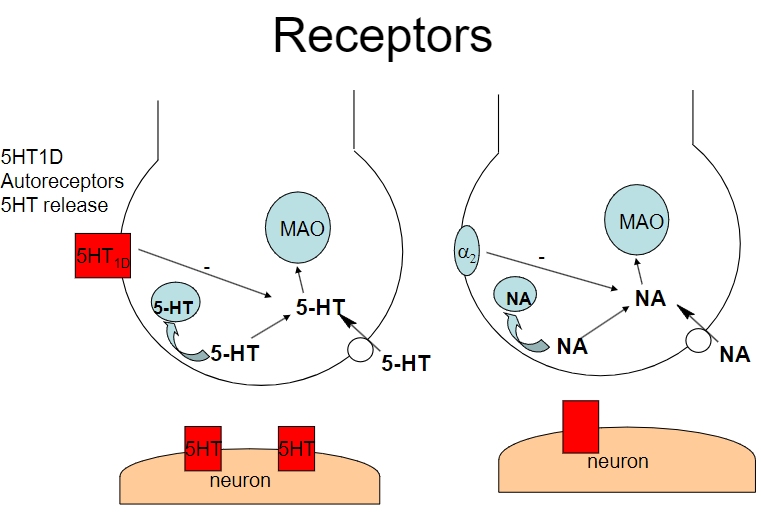
How does the regulation of noradrenaline (NA) occur in the post-synaptic and pre-synaptic neurons? (2)
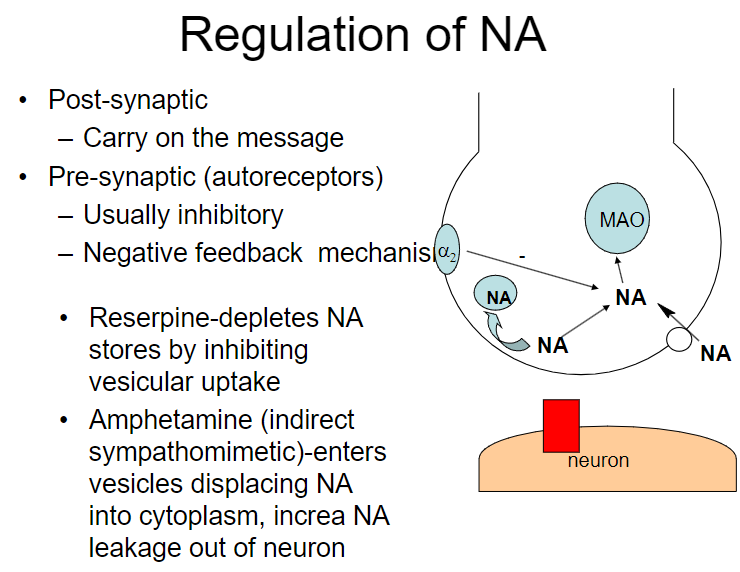
Post-synaptic: Carry on the message
Pre-synaptic (autoreceptors): Usually inhibitory, serving as a negative feedback mechanism
How do reserpine and amphetamines affect noradrenaline (NA) regulation? (2)
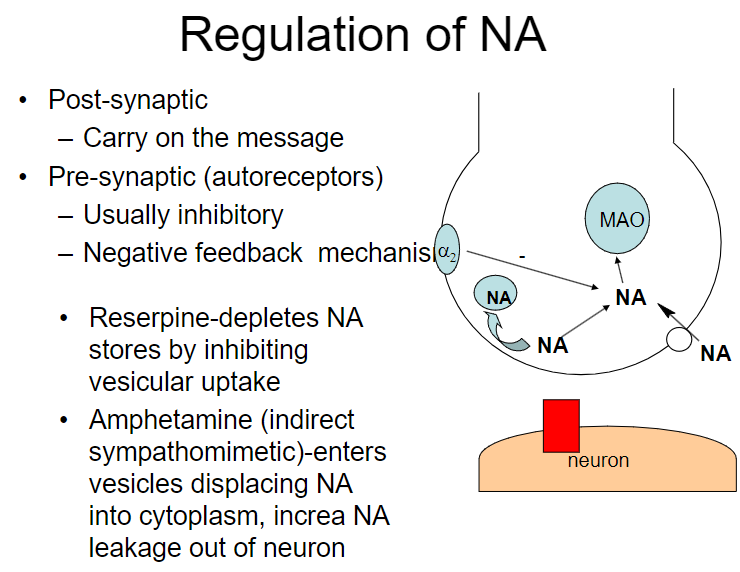
Reserpine: Depletes NA stores by inhibiting vesicular uptake
Amphetamine: Enters vesicles, displacing NA into the cytoplasm and increasing NA leakage out of the neuron
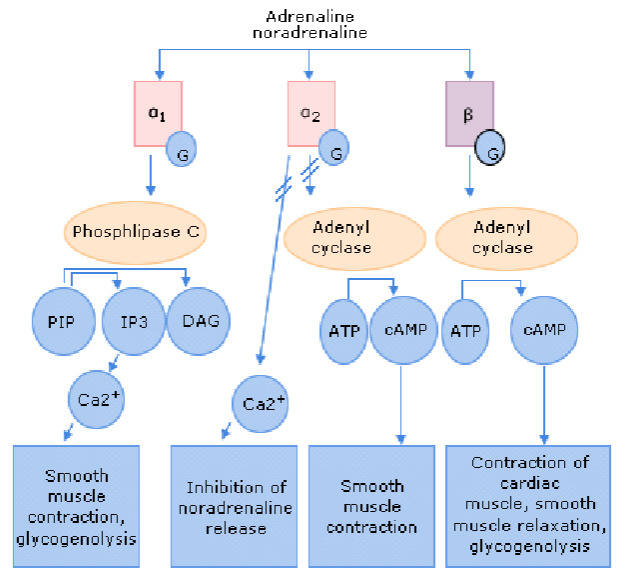
Picture demonstrating the pathways of adrenaline and noradrenalin on a/b receptors:
How do reserpine, amphetamine, and cocaine affect noradrenaline (NA) regulation? (3)
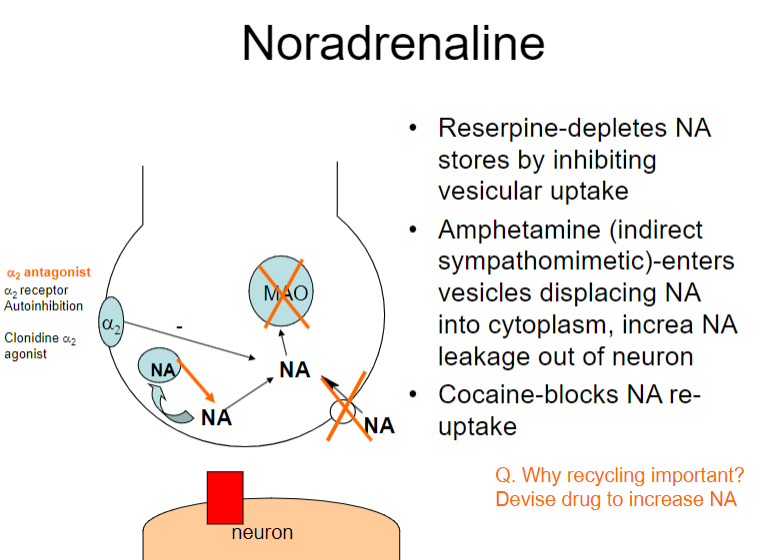
Reserpine: Depletes NA stores by inhibiting vesicular uptake
Amphetamine: Displaces NA into the cytoplasm, increasing NA leakage out of the neuron
Cocaine: Blocks NA re-uptake
What is the role of the α2 receptor in noradrenaline (NA) regulation? (1)
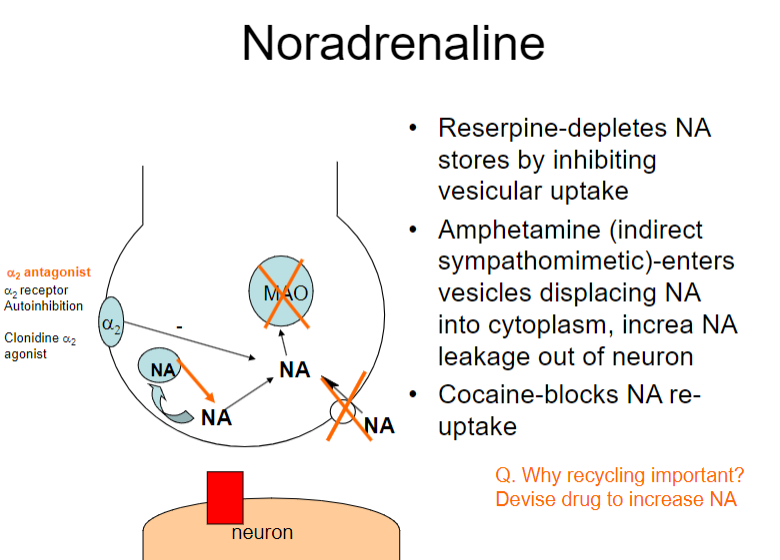
The α2 receptor mediates autoinhibition of NA release (negative feedback mechanism)
Why is recycling of noradrenaline (NA) important, and how can drugs influence this process? (2)
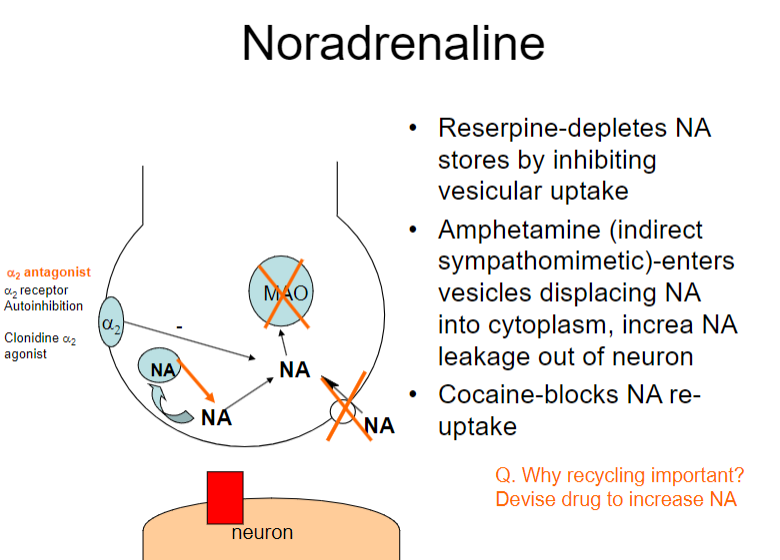
Recycling NA is important for maintaining neurotransmitter balance and ensuring effective signalling.
Drugs like α2 antagonists can increase NA release by blocking autoinhibition at the α2 receptor, potentially enhancing NA signalling.
What are the main functions of noradrenaline (NA) in the brain? (4)
Arousal and wakefulness
Exploration and mood (low NA is associated with depression)
Blood pressure regulation (e.g., antihypertensive effects of clonidine, an α2 agonist)
Addiction and gambling
What is the main action of noradrenaline (NA) and how is it terminated? (2)
Main action: Inhibitory at β receptors, excitatory at α and β receptors
Termination: Through neuronal uptake and breakdown by monoamine oxidase (MAO)
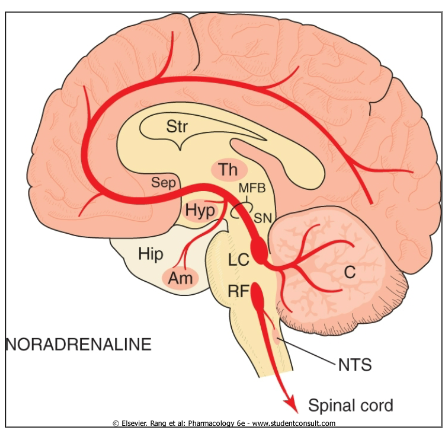
Where are noradrenergic (NAergic) neurons primarily located, and when are they most active? (2)
Main cell body: Locus coeruleus
Activity: NAergic neurons are active when the individual is awake
How does amphetamine influence noradrenaline (NA) activity? (1)
Amphetamine: Increases alertness and exploratory behavior by enhancing NA activity
What are the primary functions of dopamine (DA) and what disorders arise when DA is unregulated? (4+3)
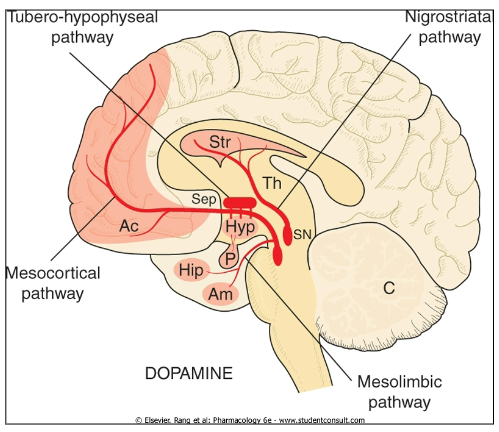
Movement
Reward
Inhibition of prolactin release
Memory consolidation
Parkinson’s Disease
Schizophrenia
Addiction
What role does dopamine (DA) play in conditions such as Parkinson’s Disease, schizophrenia, and addiction? (3)
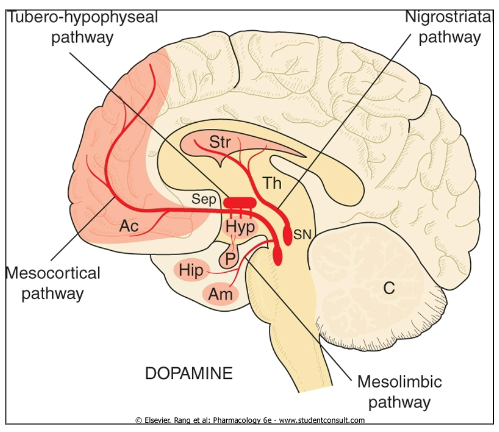
Parkinson’s Disease: Dopamine deficiency leads to motor impairment
Schizophrenia: Dysregulation of dopamine contributes to psychotic symptoms (hallucinations, delusions, etc.).
Addiction: Dopamine is involved in the brain's reward pathway, reinforcing addictive behaviours
What role does dopamine (DA) have in prolactin release and memory? (2)
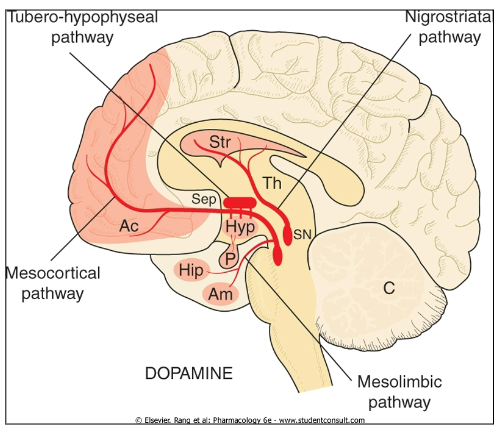
Inhibition of prolactin release: Dopamine inhibits prolactin secretion
Memory consolidation: Dopamine plays a role in consolidating memory
How is dopamine (DA) related to conditions such as emesis and ADHD? (2)
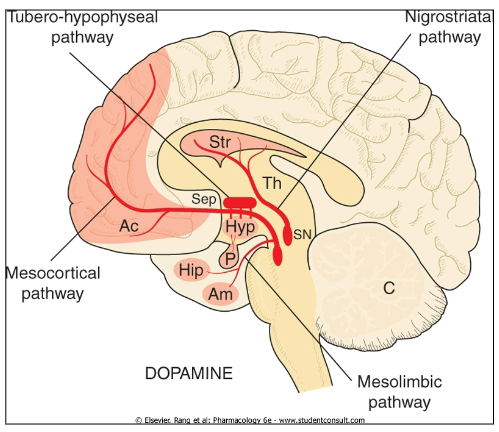
Emesis (vomiting): Dopamine is involved in the regulation of nausea and vomiting
ADHD: Dopamine dysregulation is implicated in Attention Deficit Hyperactivity Disorder
Picture demonstrating the key individuals who won a Nobel Prize in physiology or medicine in the year 2000:
Picture demonstrating dopamine regulation:
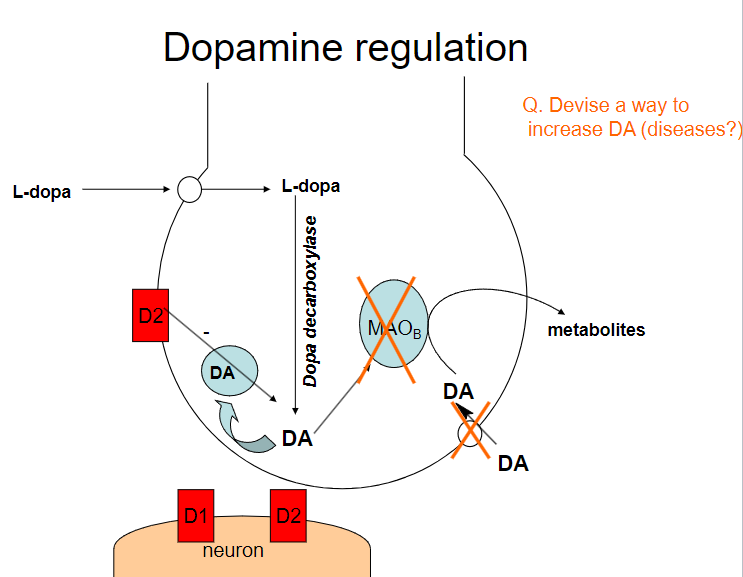
How can dopamine (DA) levels be increased? (4)
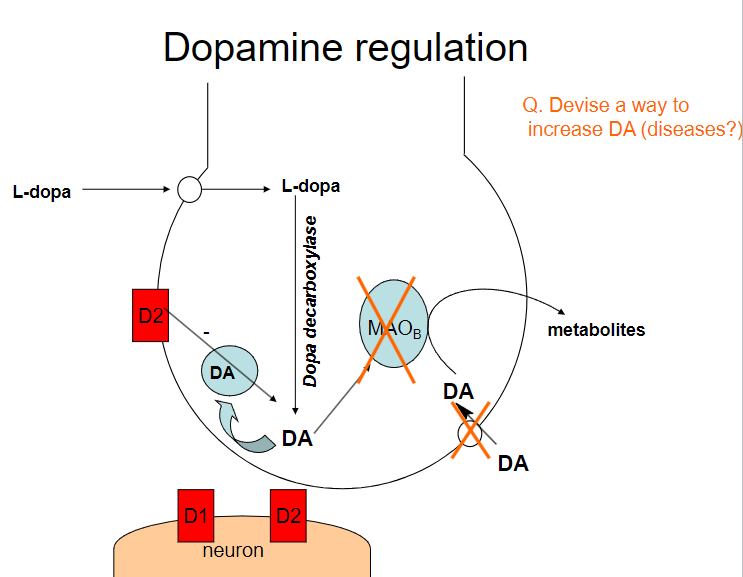
Dopamine precursors (e.g., L-DOPA): Supplementing with L-DOPA increases dopamine production
Dopamine reuptake inhibitors (e.g., cocaine, amphetamines): These drugs block the reuptake of dopamine, increasing its concentration in the synaptic cleft
Dopamine agonists (e.g., pramipexole, ropinirole): These drugs directly stimulate dopamine receptors
MAO-B inhibitors (e.g., selegiline): These inhibitors prevent the breakdown of dopamine by blocking the enzyme monoamine oxidase B (MAO-B)
What diseases could benefit from increasing dopamine (DA) levels? (3)
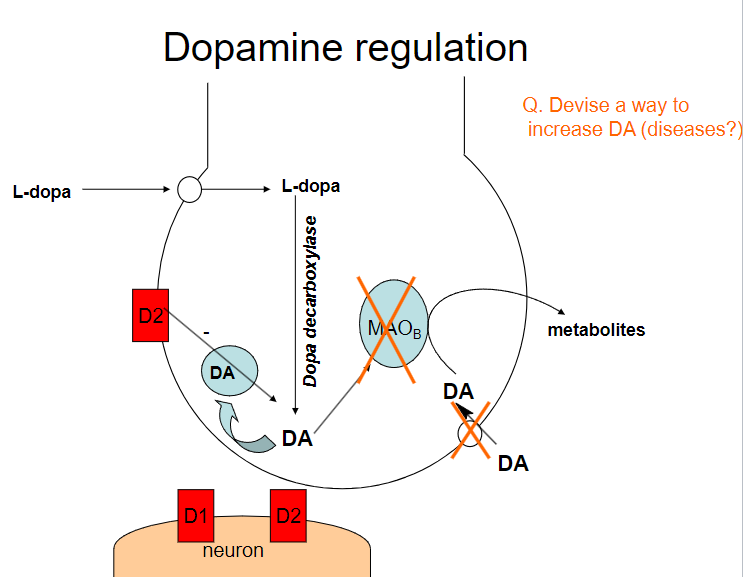
Parkinson's Disease: Increasing dopamine can help manage motor symptoms due to dopamine deficiency
Attention Deficit Hyperactivity Disorder (ADHD): Increasing dopamine activity can improve attention and focus
Depression: Some forms of depression may involve low dopamine levels, and increasing dopamine may help alleviate symptoms
What are the potential risks of increasing dopamine (DA) levels? (2)
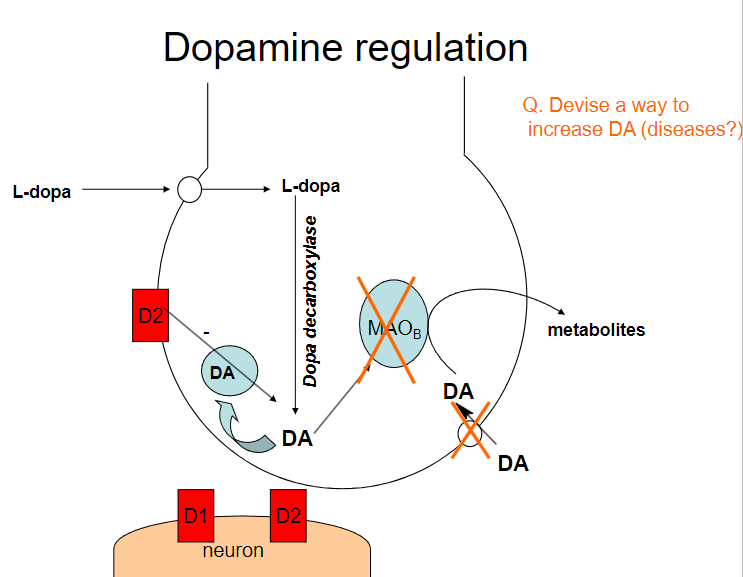
Addiction: Drugs that increase dopamine (e.g., amphetamines, cocaine) can lead to addiction due to the reinforcement of reward pathways
Psychosis: Excess dopamine activity, particularly in certain brain regions, can lead to symptoms of psychosis, such as hallucinations and delusions
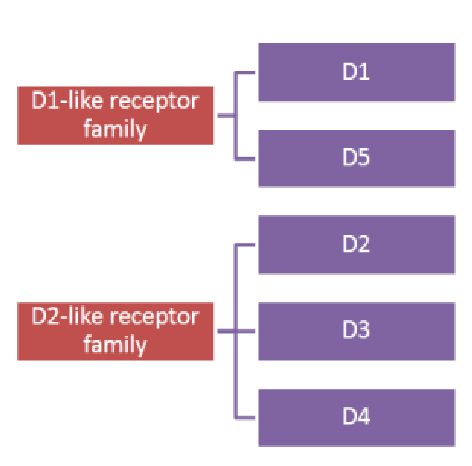
Picture demonstrating 'D' receptor families:
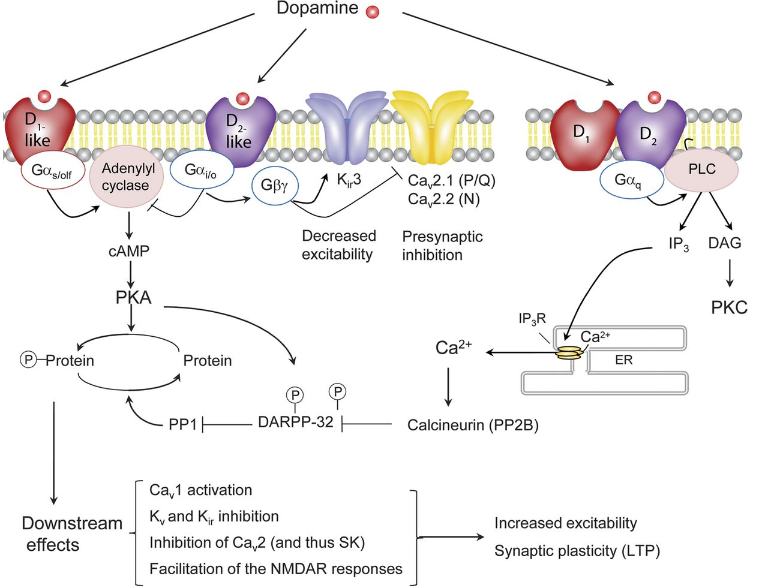
Beautiful picture demonstrating the action of Dopamine:
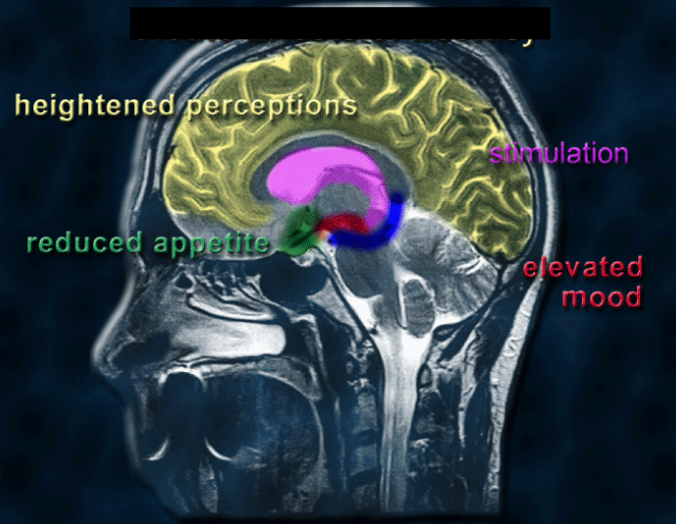
What are the effects of serotonin (5-HT) on the body? (4)
Heightened perceptions: Increased serotonin activity can enhance sensory perceptions and awareness
Stimulation: Elevated serotonin levels can promote wakefulness and alertness
Reduced appetite: Serotonin can suppress appetite, playing a role in satiety and food intake
Elevated mood: Higher serotonin levels are associated with improved mood and emotional well-being
Picture demonstrating Serotonin/5HT regulation:
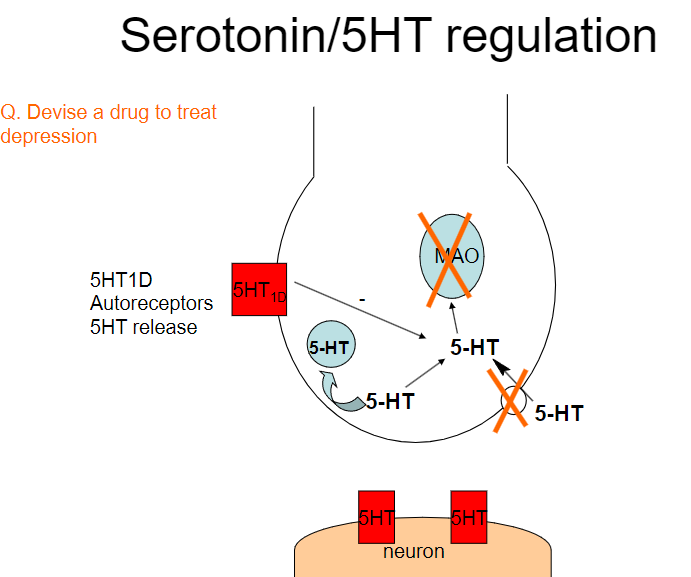
Devise a drug to treat depression based on serotonin regulation. (3)

Selective Serotonin Reuptake Inhibitors (SSRIs):
These drugs, such as fluoxetine and sertraline, inhibit the reuptake of serotonin, increasing its availability in the synaptic cleft.
By preventing serotonin from being reabsorbed by presynaptic neurons, SSRIs increase serotonin's action on postsynaptic receptors, improving mood and emotional stability.
Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs):
These drugs, like venlafaxine, inhibit both serotonin and norepinephrine reuptake, which may be more effective in some patients by addressing multiple neurotransmitter systems involved in depression.
Serotonin Agonists:
Drugs like buspirone act as partial agonists at serotonin receptors, promoting serotonin activity in areas of the brain associated with mood regulation without overstimulation.
Describe the different subtypes of 5-HT receptors and their functions. (5)
5-HT1 receptors:
Inhibitory, located primarily in the limbic system, involved in mood regulation and migraine.
5-HT2 receptors (including 5-HT2A):
Excitatory, involved in hallucinogenic effects, located in the limbic system and cortex.
5-HT3 receptors:
Excitatory, located in the medulla, associated with vomiting and nausea.
5-HT4 receptors:
Presynaptic facilitation, helps in acetylcholine release, involved in cognitive enhancement.
5-HT6 and 5-HT7 receptors:
Novel targets for cognitive functions and sleep regulation.
What is the role of serotonin (5-HT) in mood, sleep, and appetite regulation? (5)
Mood: Serotonin helps control mood. Low serotonin levels are linked to feelings of anxiety and depression.
Psychosis: Blocking serotonin (5-HT) receptors can help reduce symptoms of psychosis, which is why some antipsychotic drugs target serotonin.
Sleep/Wake: Serotonin is important for regulating sleep. Certain serotonin-blocking drugs (like 5-HT2 antagonists) can interfere with REM sleep.
Appetite: Drugs that block serotonin’s 5-HT2A receptors can increase appetite and lead to weight gain. On the other hand, some antidepressants can reduce appetite.
Pain & Migraine: Serotonin helps reduce pain and can work with opioids to manage both pain and migraines more effectively.
How is serotonin (5-HT) terminated and what are its associated disorders? (3)
Termination:
Serotonin is terminated via breakdown by monoamine oxidase (MAO) and reuptake by neuronal uptake transporters.
Associated Disorders:
Mood disorders like depression and anxiety due to imbalances in serotonin levels.
Psychosis due to serotonin receptor antagonism, often used in antipsychotic treatment.
Migraines and vomiting are linked to serotonin's actions on pain pathways and the medulla.
What are autoreceptors and what is their function in neurotransmission? (4)
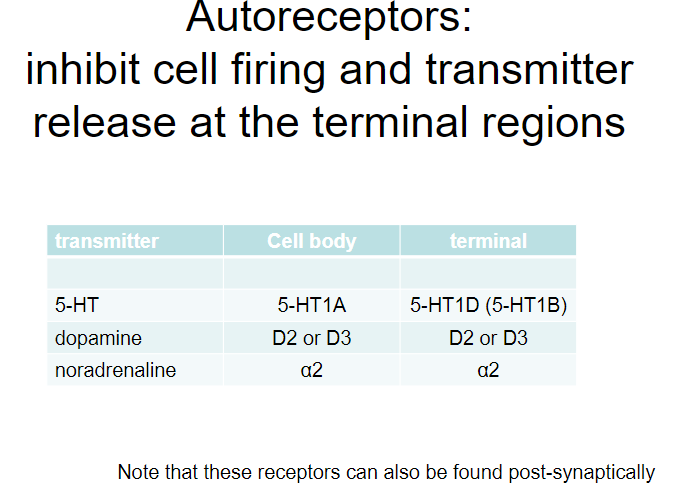
Autoreceptors:
Receptors located on the presynaptic neuron that regulate neurotransmitter release.
Function:
Autoreceptors inhibit cell firing and limit further neurotransmitter release at the terminal regions.
They act as a negative feedback mechanism to prevent excessive neurotransmitter release.
Examples of Autoreceptors:
5-HT (serotonin):
5-HT1A and 5-HT1D (5-HT1B) autoreceptors.
Dopamine (DA):
D2 and D3 autoreceptors.
Noradrenaline (NA):
α2 autoreceptors.
Picture demonstrating 5HT1D Autoreceptors and 5HT release:
What are the main transporters responsible for the reuptake of neurotransmitters and their specific locations? (5)
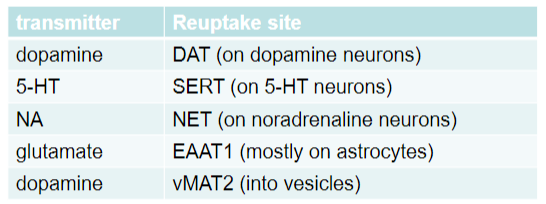
Dopamine:
Transporter: DAT
Location: On dopamine neurons
Serotonin (5-HT):
Transporter: SERT
Location: On serotonin (5-HT) neurons
Noradrenaline (NA):
Transporter: NET
Location: On noradrenaline neurons
Glutamate:
Transporter: EAAT1
Location: Mostly on astrocytes
Dopamine (vesicular transport):
Transporter: vMAT2
Location: Into vesicles
Describe the structure and function of monoamine transporters. (4)
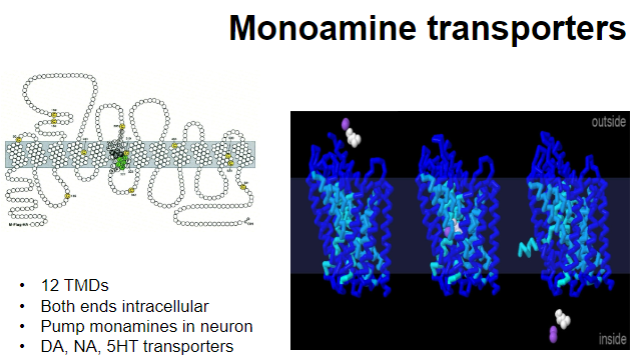
Structure:
12 Transmembrane Domains (TMDs)
Both ends are intracellular
Function:
Transport monoamines into the neuron
Involved in the reuptake of neurotransmitters like dopamine (DA), noradrenaline (NA), and serotonin (5-HT)
What are the roles of acetylcholine (ACh) in the brain, and what conditions are associated with its dysregulation? (5)
Roles of ACh:
Memory and Learning
Motor Control (e.g., striatum)
Reward
Arousal
Conditions associated with dysregulation:
Alzheimer's Disease
Pain
Addiction
Epilepsy (e.g., nAChR gene mutations)
Schizophrenia
ADHD
Depression
Anxiety
Picture demonstrating potential inhibitors of acetylcholine:

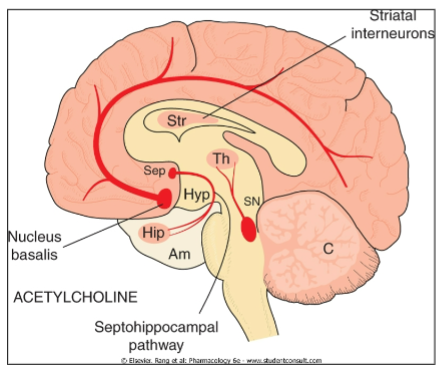
What are the key features of acetylcholine (ACh) in the brain, its receptor types, and its termination process? (6)
Key Features of ACh:
Abundant in: Basal forebrain, hippocampus, and striatum
Termination: Acetylcholinesterase (AChE) breaks down ACh in the synaptic cleft
Excitatory neurotransmitter
Receptor Types:
Nicotinic (nAChR): Ionotropic, fast action
Muscarinic (mAChR): G-protein coupled, slower action
M1 receptors: Excitatory, reduced in dementia
M2 receptors: Presynaptic inhibition (inhibits ACh release)
M3 receptors: Excitatory, affects glandular and smooth muscle (can lead to side effects)
M4 and M5 receptors: Functions not well known
What are the key functions of acetylcholine (ACh) in the brain, and which conditions is it involved in? (5+5)
Key Functions of ACh:
Arousal: Plays a role in maintaining wakefulness and alertness
Epilepsy: Mutations in nicotinic ACh receptor (nAChR) genes can contribute to epilepsy
Learning and Memory: Involved in cognitive processes, with knockout (KO) mice showing impairments
Motor Control: Muscarinic receptors inhibit dopamine (DA) release, influencing motor function
Pain and Addiction: ACh is involved in pain perception and addiction mechanisms
Neuropsychiatric Disorders:
Schizophrenia
ADHD
Depression
Anxiety
Alzheimer’s Disease
What are the functions and roles of histamine receptors? (3)
H1 receptors: Involved in arousal
H3 receptors: Presynaptic, constitutively active
Functions: Sleep/wake regulation, vomiting
What are the roles of purines in the body? (4)
Adenosine (A1, A2A/2B receptors):
Involved in sleep, pain, neuroprotection, addiction, seizures, ischemia, and anticonvulsant effects
ATP (P2X receptors):
Involved in pain, neuroprotection, and other functions
What are the functions of neuropeptides, including opioid peptides and tachykinins? (3)
Opioid Peptides:
Act on μ, δ, and κ receptors
Tachykinins:
Substance P (NK1), Neurokinin A (NK2), Neurokinin B (NK3)
Function: Primarily involved in pain regulation
What are some lipid mediators and melatonin and their functions? (4)
Lipid Mediators (Endocannabinoids):
Derived from the conversion of eicosanoids to endocannabinoids
Act on CB1 receptors to inhibit GABA and glutamate release
Functions: Involved in vomiting (CB1 agonists block it), multiple sclerosis (MS), pain, anxiety, and weight loss (e.g., rimonabant as a CB1 antagonist)
Melatonin:
Acts on MT1 and MT2 receptors
Functions: Involved in sleep regulation, circadian rhythmicity, and used as an agonist for jet lag and insomnia
What are the mechanisms of action for amphetamine-like drugs such as methylphenidate and MDMA? (2)
Release cytosolic monoamines (DA)
Prolonged use can lead to neurotoxicity, degeneration of amine-containing nerve terminals, and cell death
What are the pharmacological effects of amphetamine-like drugs such as methylphenidate and MDMA? (7)
Increased alertness and locomotor stimulation
Euphoria / excitement
Stereotyped behaviour
Anorexia
Reduced physical and mental fatigue (helps with monotonous tasks)
Peripheral sympathomimetic actions: Increased blood pressure and decreased gastric motility
Improved confidence and lack of tiredness
What are the therapeutic uses of amphetamine-like drugs such as methylphenidate and MDMA? (3)
Treatment for ADHD (e.g., methylphenidate)
Appetite suppressants
Treatment for narcolepsy
What is the mechanism of action of cocaine? (1)
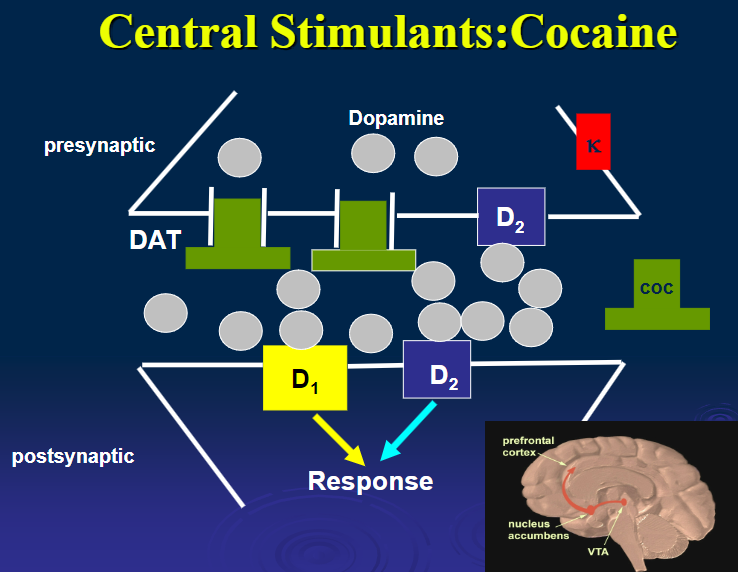
Blocks catecholamine reuptake, leading to increased DA and stimulant effects.
What are the pharmacological effects of cocaine? (5)
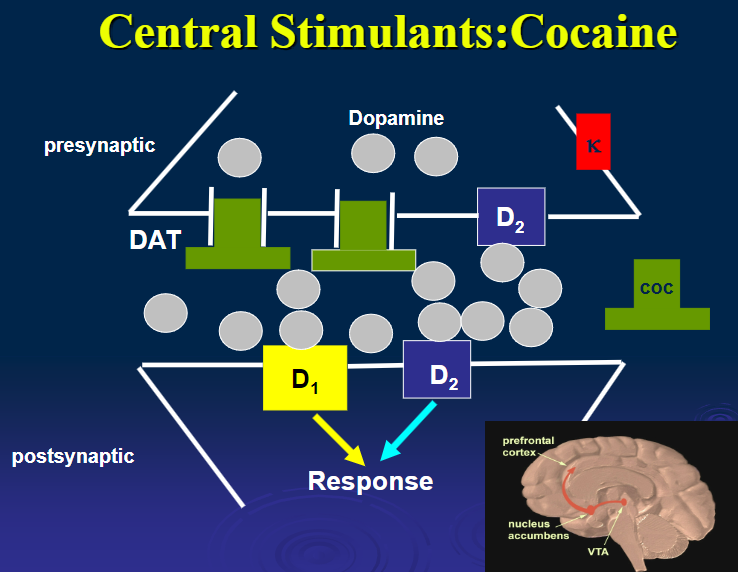
Euphoria
Locomotor stimulation
Fewer stereotyped behaviours compared to amphetamine
Heightened pleasure
Lower tendency for delusions, hallucinations, and paranoia
What are the pharmacokinetics of cocaine? (3)
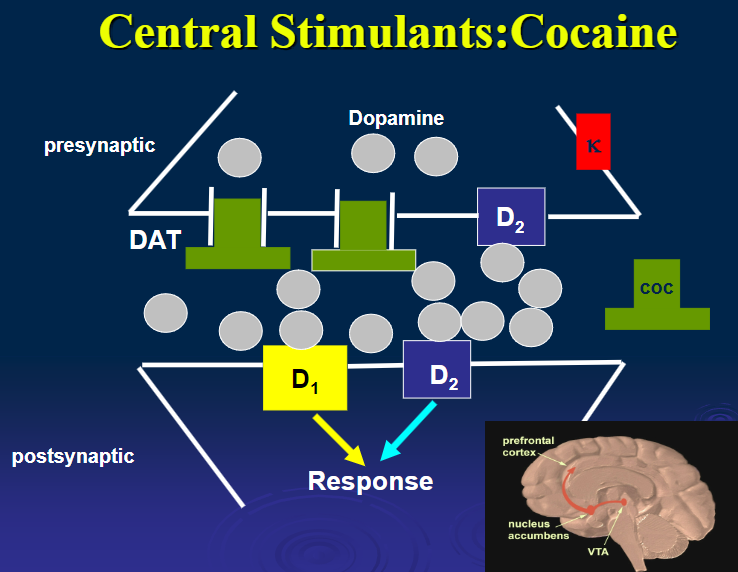
Cocaine is available as a HCl salt, and can be inhaled or administered i.v.
Nasal inhalation is less intense but can cause necrosis of nasal mucosa.-Freebase form (‘crack’), smoked, as intense as i.v route
What is the mechanism of action of MDMA (ecstasy)? (2)
Inhibits monoamine transporters, mainly 5-HT (serotonin).
Causes a large increase in 5-HT, followed by depletion.
What are the effects of MDMA (ecstasy) on serotonin and dopamine? (3)
Increase in 5-HT linked to psychotomimetic effects.
Increase in DA (dopamine) linked to euphoria, followed by rebound dysphoria.
How do LSD and Psilocybin work? (1)

They produce hallucinogenic effects by acting on 5HT2 receptors, they are agonists of serotonin.
What is neuromodulation? (1)
It is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons.
What are neuromodulators? (1)
Neuromodulators are neurotransmitters that diffuse through neural tissue to affect slow-acting receptors of many neurons.
What is the Locus Coeruleus? (2)
It is a nucleus in the pons of the brainstem involved in the physiological responses to stress and panic.
It is the site of brain synthesis of noradrenaline.
What are the Raphe Nuclei? (2)
A collection of nuclei of neurons in the brainstem.
They produce serotonin.
What is the Basal Forebrain Complex? (2)
Located in the forebrain in front of and below the striatum.
Includes structures like the nucleus accumbens, nucleus basalis, diagonal band of Broca, substantia innominata, and the medial septal nucleus, and is rich in cholinergic neurons.
What are hallucinogens? (1)
Compounds or drugs that produce hallucinations, such as LSD and Psilocybe.
What are stimulants? (2)
A drug that produces a temporary increase in psychomotor activity.
Often induces feelings of euphoria, alertness, and self-confidence, examples include cocaine and amphetamine.