What is motivation and how is it related to the hypothalamus? (5)
Motivation is the driving force behind behavior and can be influenced by:
Physical needs (e.g., hunger, thirst)
Wanting and liking (emotional drivers for behavior)
The hypothalamus helps maintain homeostasis by regulating three key functions:
Endocrine secretion: Hormonal control of bodily functions.
Autonomic nervous system: Controls involuntary functions like heart rate and digestion.
Emotions and drive/behavior: Motivates actions such as eating and drinking.
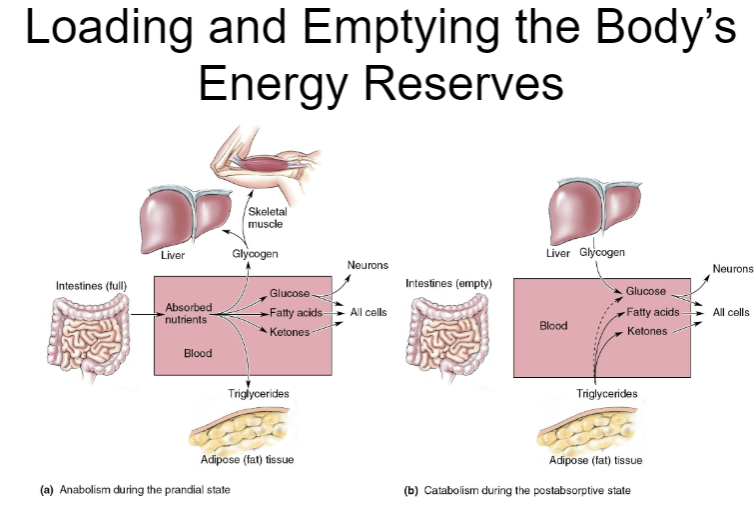
Picture demonstrating Loading and Emptying the Body’sEnergy Reserves:
Picture demonstrating The long-term regulation of feeding behaviour and body fat:
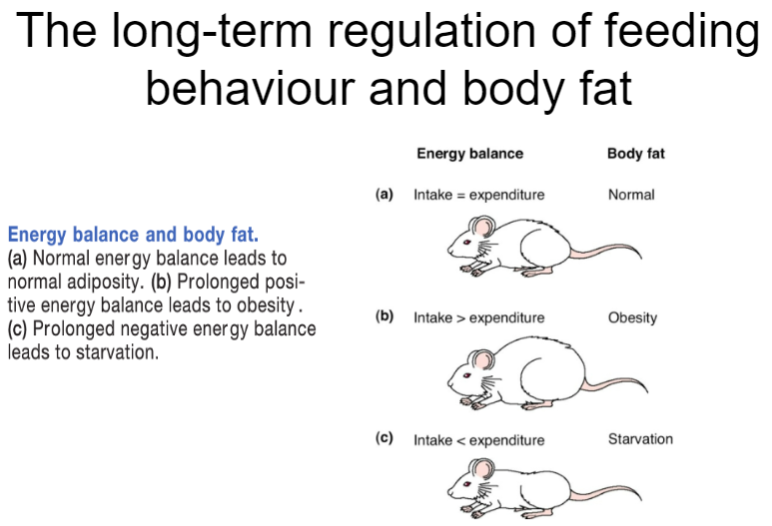
How is body weight regulated long-term? (4)
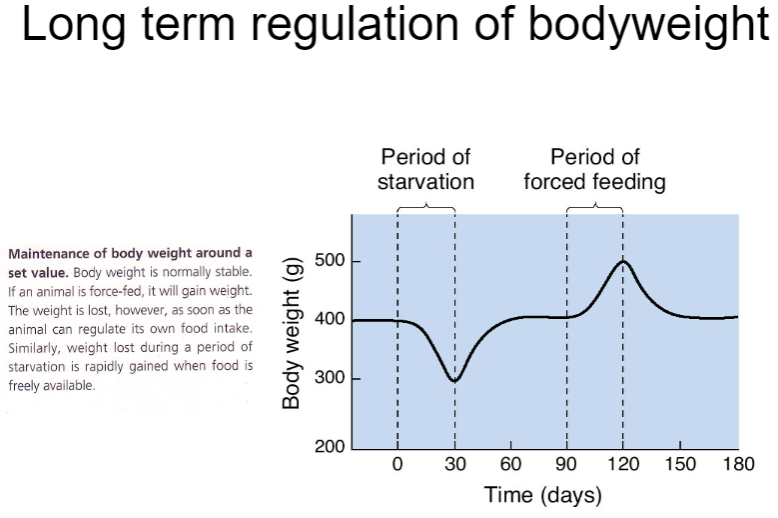
Long-term body weight regulation involves complex hormonal and neural mechanisms.
Leptin: Produced by fat cells, signals the brain to decrease appetite and increase energy expenditure when fat stores are high.
Insulin: Helps regulate glucose levels and also plays a role in long-term body weight control by influencing fat storage.
The hypothalamus integrates these signals to maintain stable body weight by adjusting hunger, metabolism, and energy use.
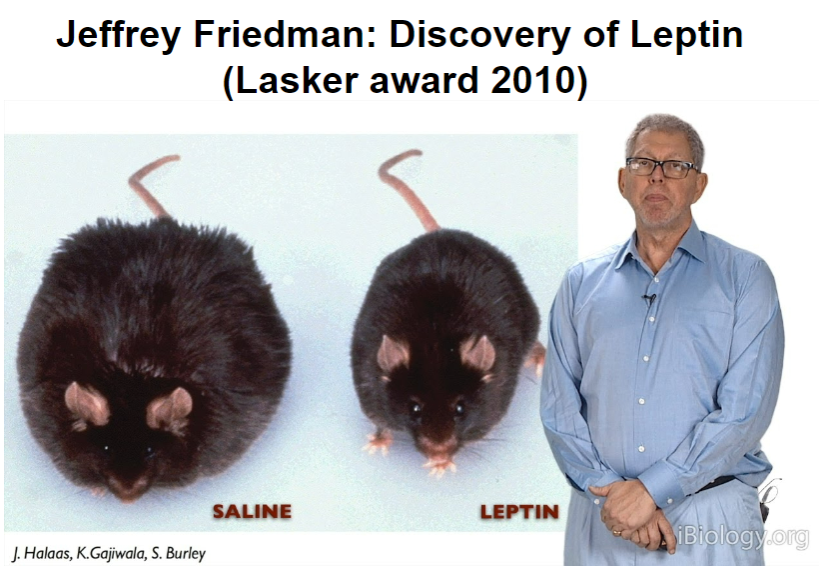
What is parabiosis and how does it affect body weight in ob/ob mice? (4)
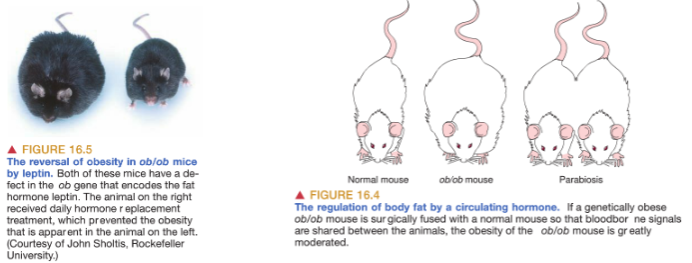
Parabiosis refers to the sharing of blood circulation between two animals, allowing blood-borne signals to be shared.
These shared signals can affect the hypothalamus, which regulates body weight.
In the case of ob/ob mice, which are genetically obese due to their fat cells not producing leptin (a hormone that inhibits food intake),
When connected to a normal mouse that produces leptin, the ob/ob mouse experiences a reduction in obesity, suggesting leptin’s role in regulating body weight.
Picture demonstrating Jeffrey Friedman: Discovery of Leptin:
How does leptin regulate eating behaviour through the arcuate nucleus? (4)
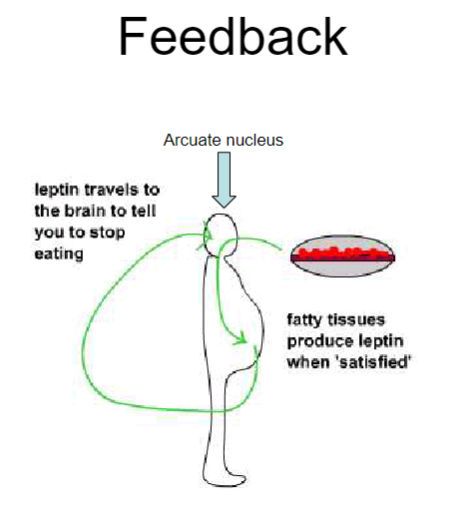
Leptin is produced by fatty tissues when energy stores are 'satisfied' (i.e., when fat stores are high).
It travels to the arcuate nucleus in the brain, which is involved in regulating hunger and energy balance.
When leptin levels are high, the arcuate nucleus signals the brain to reduce hunger and stop eating.
This feedback loop helps maintain stable body weight by preventing overeating when fat stores are sufficient.
What structures are found in the frontal section of the hypothalamus and their roles? (4)
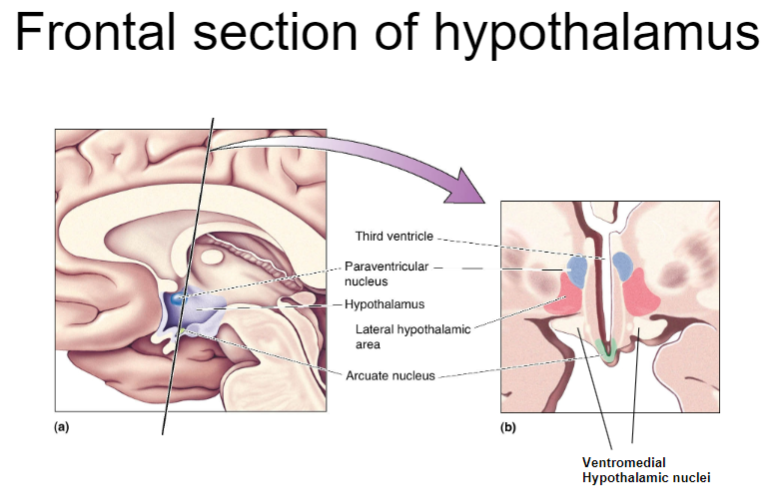
The arcuate nucleus: Involved in regulating hunger, satiety, and energy balance.
The ventromedial hypothalamus (VMH): Plays a key role in satiety, signaling to stop eating when energy stores are sufficient.
The lateral hypothalamus (LH): Involved in hunger signals and the motivation to eat.
The preoptic area: Regulates thermoregulation and sleep patterns, also influencing feeding behavior.
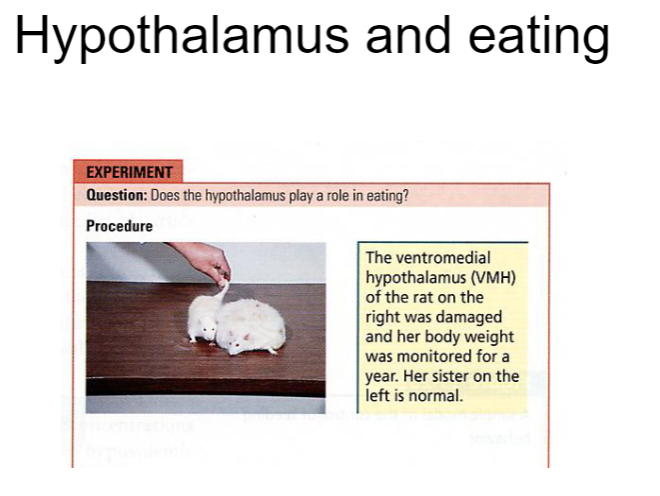
What are the effects of a VMH lesion on homeostasis, body weight, and food intake? (3)
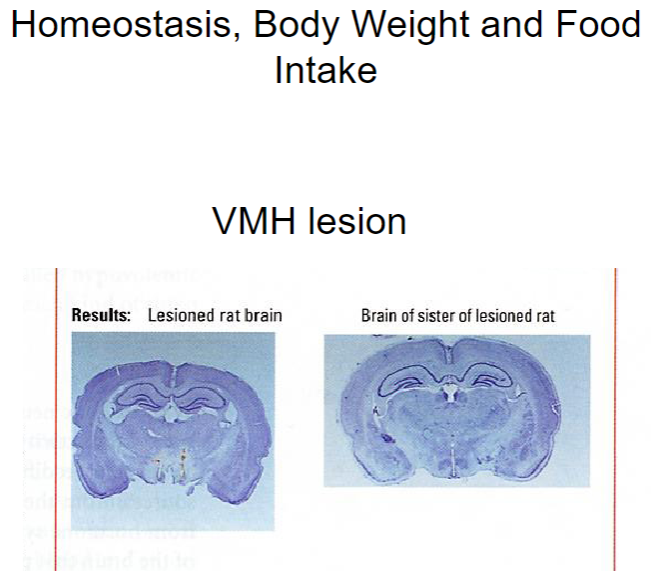
VMH (ventromedial hypothalamus) lesion: Disrupts the body's ability to regulate satiety, leading to overeating and weight gain.
This lesion causes the animal to lose the ability to sense when it is full, impairing homeostasis and energy balance.
Increased food intake: Without the satiety signal from the VMH, animals will continue to eat, leading to obesity and higher body weight.
How does a VMH lesion affect body weight? (3)
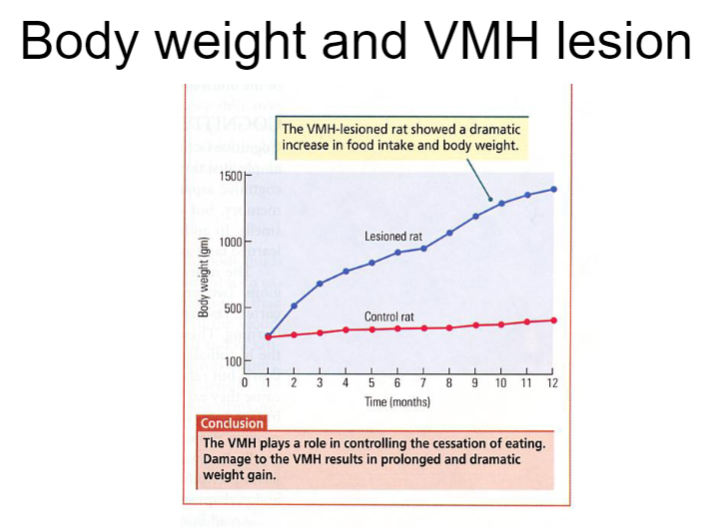
A VMH lesion disrupts the regulation of hunger and satiety, leading to an inability to properly control food intake.
As a result, animals with a VMH lesion will experience increased food consumption, often leading to significant weight gain.
Despite overeating, the body’s energy balance remains impaired, and the animal's weight becomes higher than normal, as the lesion prevents proper homeostatic regulation of body weight.
Picture demonstrating role of Hypothalamus and eating:
What are the effects of lesions in the VMH and LH on food intake? (4)
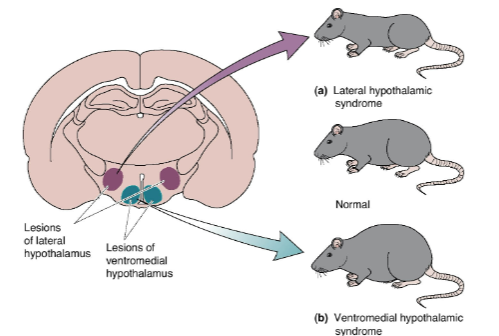
Lateral hypothalamic syndrome (LH lesion): Results in diminished appetite for food, leading to anorexia.
Ventromedial hypothalamic syndrome (VMH lesion): Leads to overeating and obesity due to impaired satiety signaling.
Both syndromes are related to disruptions in leptin signaling, which plays a key role in regulating hunger and energy balance.
The LH is typically involved in hunger signals, while the VMH is responsible for signaling satiety.
What is the role of the arcuate nucleus in feeding control and how does leptin affect it? (4)
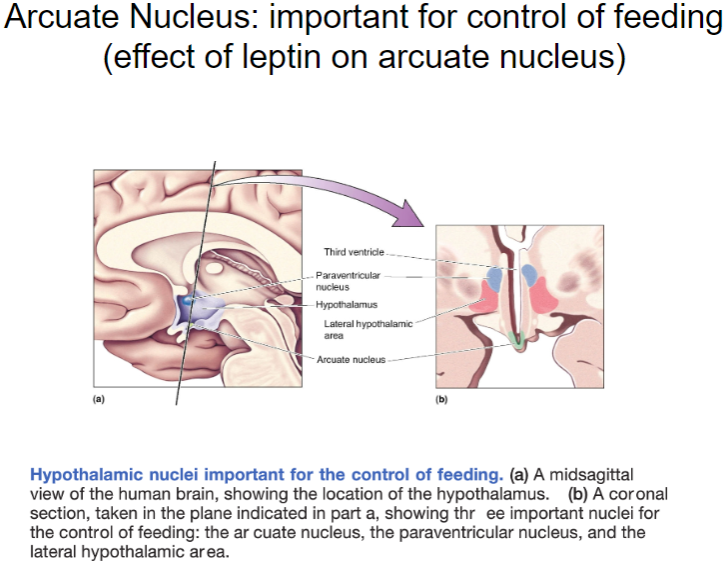
The arcuate nucleus in the hypothalamus plays a crucial role in regulating food intake and energy balance.
Leptin, produced by fat cells, acts on the arcuate nucleus to decrease hunger and reduce food intake when fat stores are sufficient.
Leptin signals the arcuate nucleus to activate neurons that promote satiety and suppress hunger.
This regulation helps maintain body weight and energy homeostasis by coordinating feeding behavior in response to leptin levels.
What is the anorexic response to the injection of CART/αMSH and how does it mimic leptin? (3)
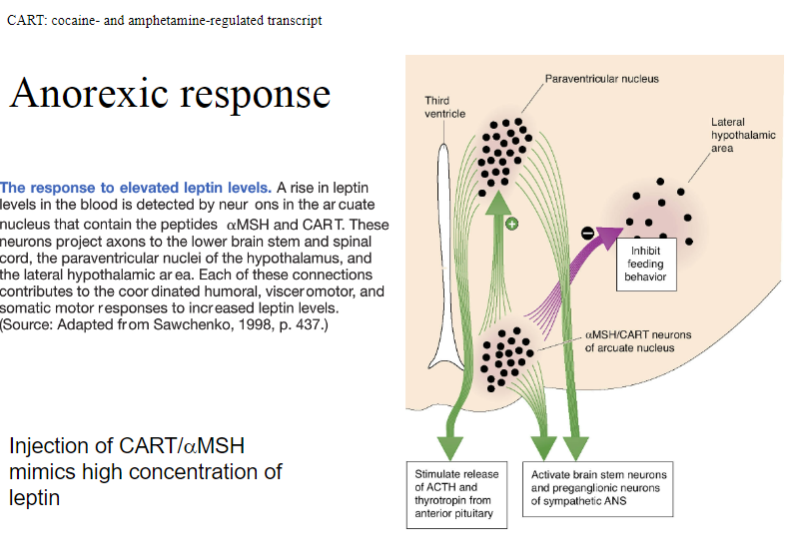
Injection of CART (cocaine- and amphetamine-regulated transcript) or αMSH (alpha-melanocyte-stimulating hormone) induces an anorexic response, reducing food intake.
These substances mimic high leptin concentrations by signaling the brain to reduce hunger and increase satiety.
Both CART and αMSH activate pathways similar to leptin, leading to a decrease in appetite and food consumption.
What is the response to elevated leptin levels and how does it affect appetite and behavior? (5)
Elevated leptin levels activate arcuate nucleus neurons that release αMSH (alpha-melanocyte-stimulating hormone) and CART (cocaine- and amphetamine-regulated transcript) peptides.
These are anorectic peptides, meaning they diminish appetite.
The arcuate neurons project to several brain regions to coordinate responses:
Paraventricular nucleus: Triggers the humoral response (hormonal regulation).
Intermediolateral gray matter of spinal cord: Coordinates the visceromotor response (visceral reactions).
Lateral hypothalamus: Controls the somatic response (behavioral actions, such as physical movements related to food intake).
What is the orexigenic response to the injection of NPY/AgRP and how does it mimic low leptin concentrations? (3)
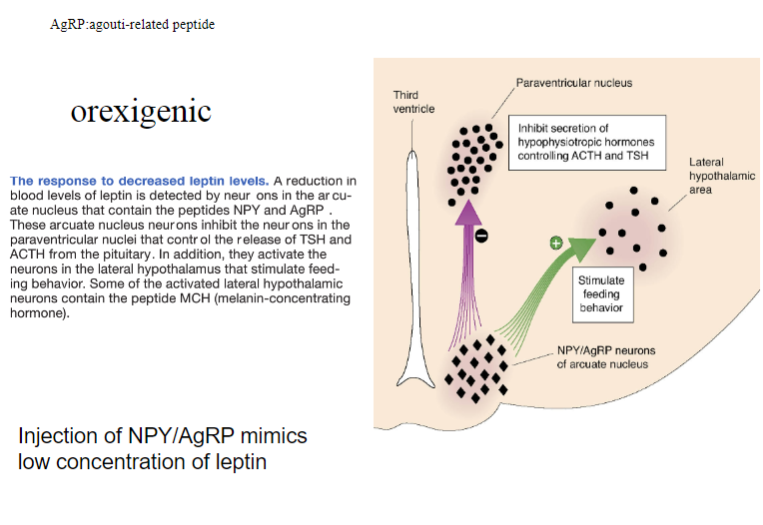
Injection of NPY (Neuropeptide Y) and AgRP (Agouti-related peptide) induces an orexigenic response, meaning it increases appetite.
These substances mimic low leptin concentrations by signaling the brain to promote hunger and increase food intake.
NPY and AgRP work by inhibiting the effects of anorectic peptides (like αMSH) and activating pathways that drive hunger.
What is the competition for activation of the MC4 receptor? (3)
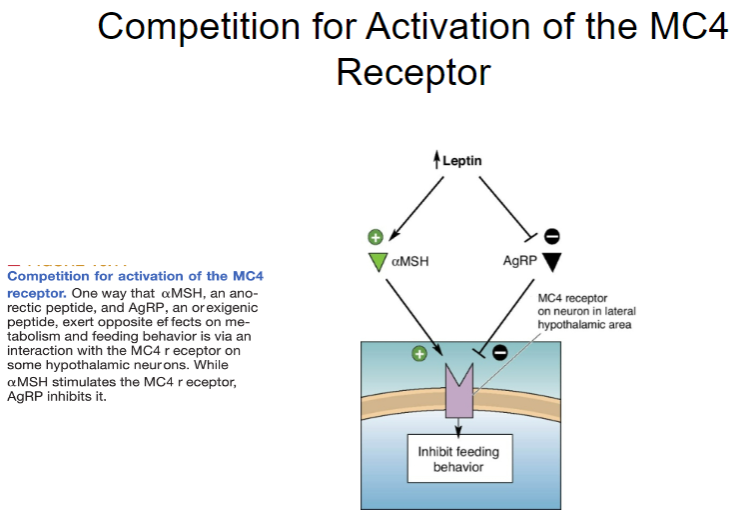
The MC4 receptor (Melanocortin 4 receptor) is involved in regulating appetite and energy balance.
αMSH (alpha-melanocyte-stimulating hormone), an anorectic peptide, activates the MC4 receptor to decrease food intake.
AgRP (Agouti-related peptide), an orexigenic peptide, competes with αMSH for activation of the MC4 receptor, blocking its anorectic effect and promoting hunger instead.
What are the two key peptides in the lateral hypothalamus that control feeding behavior and their effects? (4)
Melanin-concentrating hormone (MCH):
Found in LH neurons, MCH stimulates feeding behavior.
It has widespread connections throughout the brain and prolongs food consumption, encouraging continued eating.
Orexin:
Also present in LH neurons, orexin has widespread cortical connections.
It plays a key role in initiating meals, promoting the start of eating behavior.
Picture demonstrating Anorectic and Orexigenic Peptidesof the hypothalamus:
How does the hypothalamus regulate body weight and food intake, and what are the effects of disruptions in this regulation? (4)
The hypothalamus plays a crucial role in regulating feeding behavior, both in the long term and through the action of hypothalamic peptides and leptin, which is produced by adipose (fat) tissue.
Leptin helps to accurately regulate food intake and body weight by signaling the hypothalamus to adjust hunger and satiety.
Disruption of this regulatory system can lead to various eating disorders, including:
Hyperphagia (excessive eating).
Anorexia (lack of appetite).
Bulimia nervosa (binge eating followed by compensatory behavior).
What factors influence the short-term regulation of feeding behavior? (3)
Time since the last meal: The longer it has been since the last meal, the stronger the motivation to eat.
Amount of food already eaten: The more food already consumed, the less hungry the individual may feel.
Type of food consumed: The composition of the meal (e.g., fats, proteins, carbohydrates) can affect the feeling of fullness and influence hunger levels.
What is satiety and how does it affect eating behaviour? (4)
Satiety is the feeling of fullness and suppression of hunger that occurs after a meal.
It influences how soon and how much you eat next, by signaling when to stop eating.
Satiety is triggered by a series of bodily signals that begin when food or drink is consumed and continue as it enters the gut, is digested, and absorbed.
These signals involve complex interactions between the gut, hormones, and brain to regulate hunger and food intake.
Picture demonstrating a Model for Short-Term Regulation of Feeding:
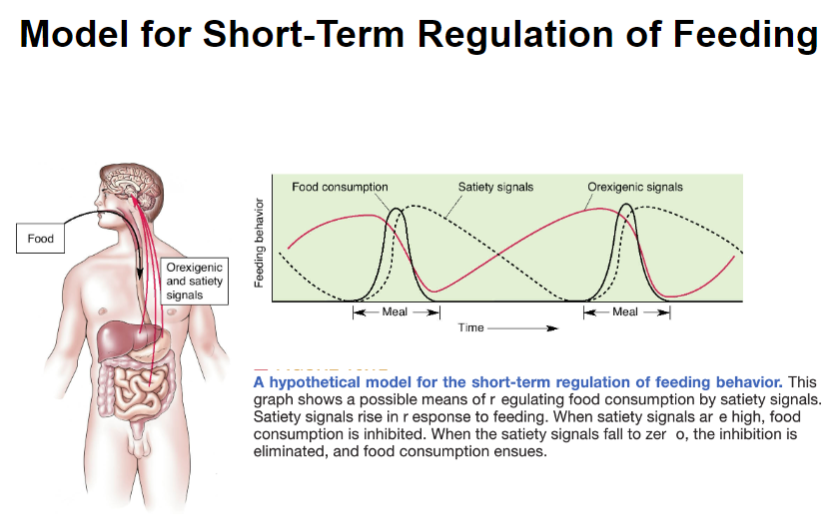
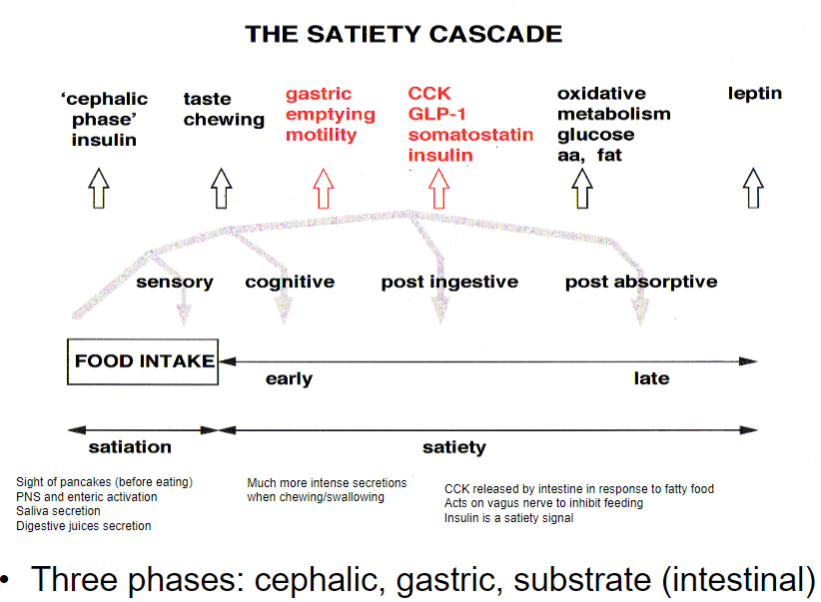
What is the satiety cascade, and how does it regulate eating? (3)
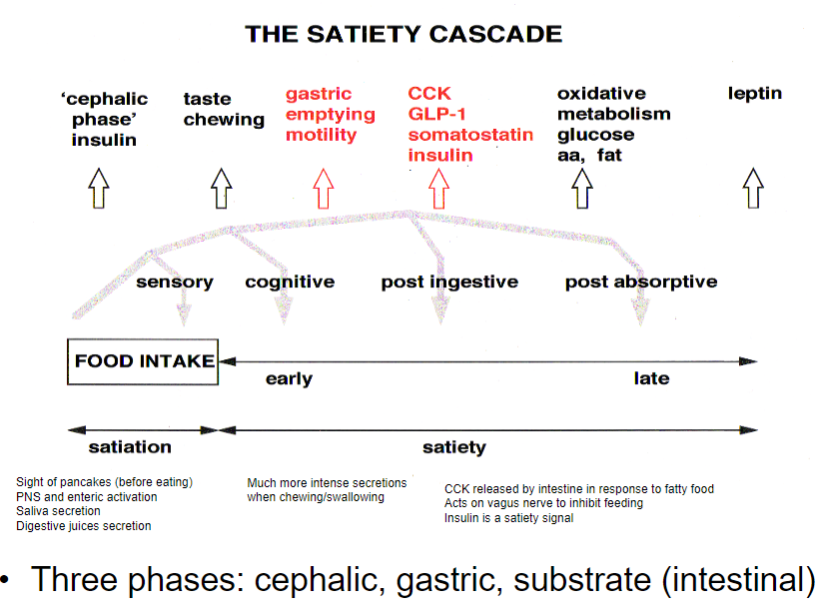
Cephalic phase: The sight or smell of food activates the PNS and enteric nervous system, leading to saliva and digestive juice secretion.
Gastric phase: As food is chewed and swallowed, CCK is released in response to fatty food, acting on the vagus nerve to inhibit feeding.
Substrate (intestinal) phase: Insulin is released as a satiety signal, indicating absorption of nutrients and reducing hunger.
What happens during the cephalic phase of feeding regulation? (4)
Hunger signals are triggered by the release of ghrelin when the stomach is empty.
Ghrelin activates NPY/AgRP-containing neurons in the arcuate nucleus of the hypothalamus, stimulating appetite.
Removal of ghrelin-secreting cells in the stomach can result in loss of appetite, indicating the importance of ghrelin in initiating hunger.
What signals contribute to the termination of a meal and satiety? (3)
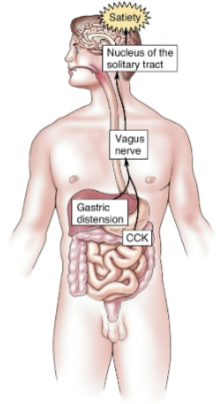
Gastric distension signals the brain via the vagus nerve, indicating fullness.
This works synergistically with CCK (cholecystokinin) released in the intestines in response to certain foods.
Insulin is released by β cells of the pancreas, promoting satiety by acting on the arcuate nucleus of the hypothalamus.
Why do we eat and how are liking and wanting mediated? (4)
Hedonic aspect: We eat because we like food, which is driven by the pleasure it provides.
Drive reduction: We eat because we want food to satisfy biological needs.
Separate brain circuits: Liking and wanting are mediated by separate brain circuits, with different mechanisms for each.
Dopaminergic system: The dopamine system is involved in wanting and craving, playing a role in the motivation to eat, though it may also influence liking.
What are positive and negative reinforcement? (4)
Positive reinforcement: Anything added after a behavior that makes it more likely for the behavior to occur again in the future.
Negative reinforcement: A response or behavior is strengthened by stopping, removing, or avoiding a negative outcome or aversive stimulus.
What is the role of D2 receptors in obesity? (3)

D2 receptors are part of the dopaminergic system and play a role in motivation and reward.
Reduced D2 receptor availability has been linked to increased food intake and obesity, as it can impair the brain’s ability to process reward signals from food.
Lower D2 receptor activity may lead to an increased tendency to seek out and consume high-reward foods, contributing to overeating.
What is the role of D2 receptors in drug abusers? (3)
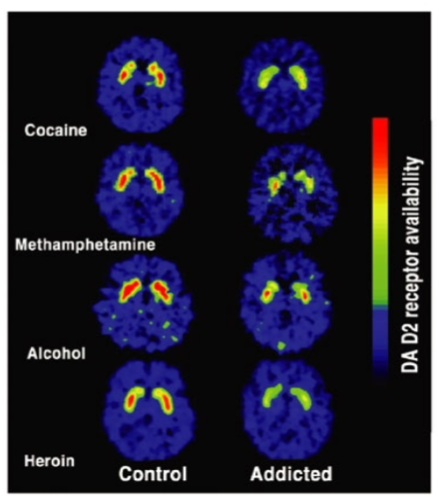
D2 receptors are involved in the brain's reward system and influence motivation and pleasure.
Reduced D2 receptor availability in drug abusers is linked to impaired reward processing, leading to a diminished ability to experience pleasure from natural rewards.
This reduction in D2 receptor activity may increase vulnerability to substance abuse, as individuals may seek drugs to compensate for the lack of natural reward response.
What are the key neural circuits involved in reinforcement? (3)
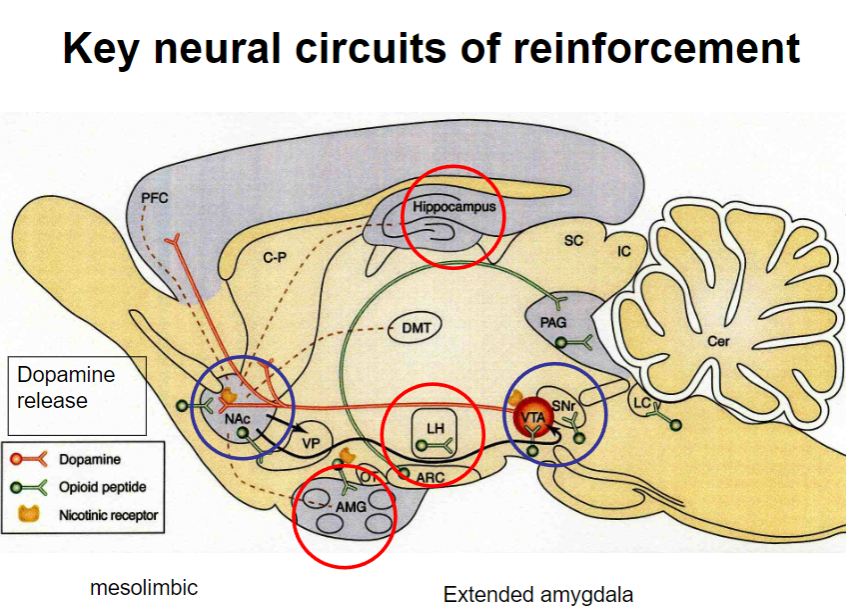
Extended amygdala: Plays a critical role in the processing of emotions and motivation, particularly in reinforcement.
Mesolimbic pathway: Involves the dopaminergic system, linking the ventral tegmental area (VTA) to the nucleus accumbens, and is key in reinforcement and reward.
Dopamine release: The mesolimbic pathway is activated, releasing dopamine in response to rewarding stimuli, strengthening the likelihood of a behavior being repeated.
What is operant behaviour and self-stimulation? (3)
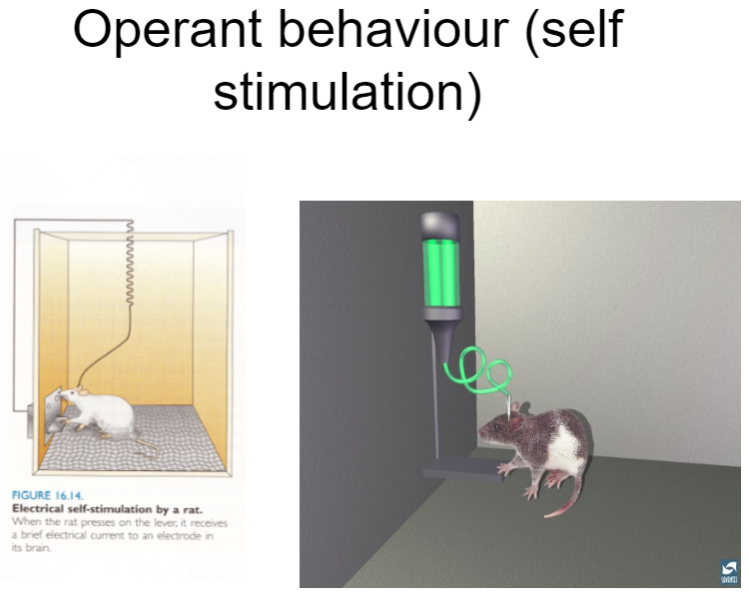
Operant behaviour: A type of behaviour that is influenced by consequences, where behaviours are reinforced or punished to increase or decrease their occurrence.
Self-stimulation: A form of operant behaviour where individuals engage in activities (like pressing a lever) to stimulate dopamine release, often in brain regions like the nucleus accumbens, leading to pleasurable sensations.
Reinforcement: Self-stimulation is a form of reinforcement, where the behaviour of seeking stimulation is repeated due to the positive feedback of dopamine release.
What is microdialysis and how is it used? (3)

Microdialysis: A technique used to measure neurotransmitter release in living organisms (in vivo) by sampling extracellular fluid in the brain.
Neurotransmitter analysis: It allows researchers to track changes in neurotransmitter levels in response to various stimuli or conditions.
Association with behavior: Microdialysis is often used to study how changes in neurotransmitter release are associated with behavioral parameters, helping to understand the neurochemical basis of behavior.
What is the role of dopamine in reinforcement? (3)
Dopamine release in the nucleus accumbens is correlated with motivation to pursue rewards, but not with the actual liking or hedonic pleasure of the reward.
Dopamine is also released in anticipation of a reward, driving behavior aimed at achieving the goal.
In addition to reinforcement, dopamine is also involved in movement, as seen in disorders like Parkinson’s disease where dopamine pathways are impaired.
How is serotonin involved in food and mood regulation? (4)
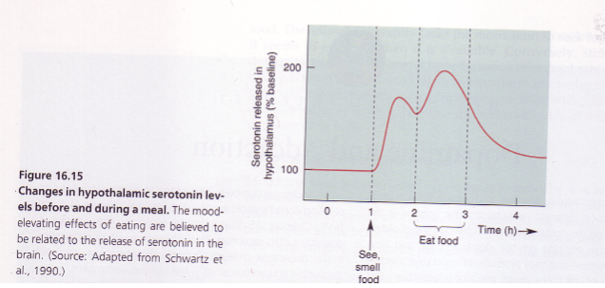
Serotonin (5HT) in the hypothalamus: Plays a crucial role in regulating both mood and food intake.
Rises in anticipation of food: Serotonin levels increase when anticipating food, signaling hunger and influencing behavior.
Spike during meals: Serotonin levels rise especially when consuming carbohydrates, which may contribute to feelings of satiety and well-being.
Association with eating disorders: Low serotonin levels are linked to depression and eating disorders like anorexia nervosa and bulimia, where mood and food intake are disrupted.
How does the hypothalamus integrate with the cortex and periphery? (4)
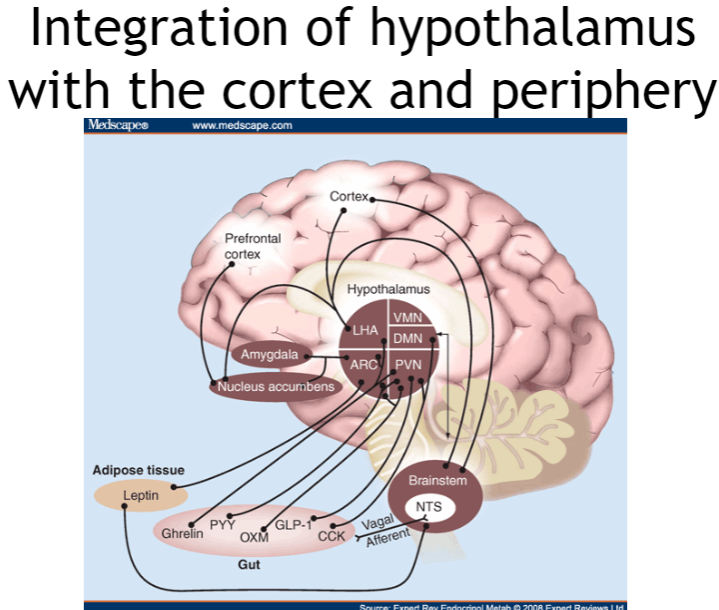
Cortex and hypothalamus: The cortex regulates higher-order functions, influencing the hypothalamus to control behaviors like feeding and homeostasis.
Periphery and hypothalamus: The hypothalamus receives feedback from the body (e.g., hormones, sensory signals) to manage hunger, satiety, and temperature.
Bidirectional communication: It adjusts behaviors like eating and drinking in response to changes in the body and environment.
Homeostasis control: The hypothalamus integrates signals from both the cortex and periphery to maintain balance, controlling autonomic functions and emotions.
Ozempic (Semaglutide) for Weight Loss: Mechanism and Impact (5)
GLP-1 Agonist: Ozempic (Semaglutide) is a GLP-1 agonist, similar to the satiety hormone GLP-1, which regulates appetite by interacting with brain appetite centers.
Action on Hypothalamus: It stimulates GLP-1 receptors in the hypothalamus, promoting anorexigenic signals (POMC, CART, αMSH) and inhibiting orexigenic signals (NPY, AgRP).
Vagal Pathway: Acts through vagal afferents on brainstem nuclei (NTS) that signal the hypothalamus to reduce appetite.
Long-term Weight Loss: Clinical studies show significant long-term weight loss effects.
Popularity: Ozempic has become a popular and effective medication for weight loss due to its significant impact on appetite regulation.
Picture demonstrating the homeostatic regulation of feeding:
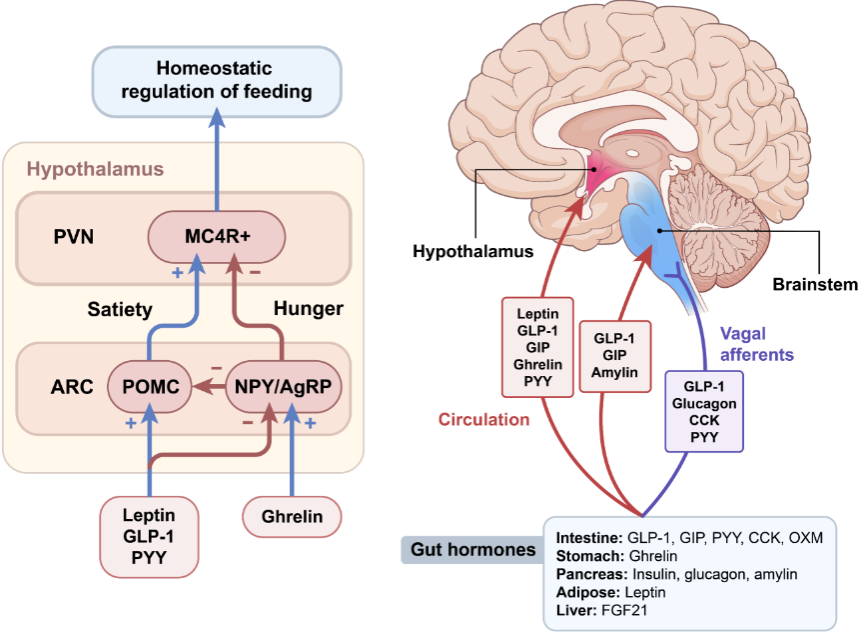
What is motivated behaviour and why is it important? (2)
Motivated behaviour is the driving force or energizing factor behind actions pursued to achieve a goal.
It is a fundamental aspect of how we interact with the world and each other.
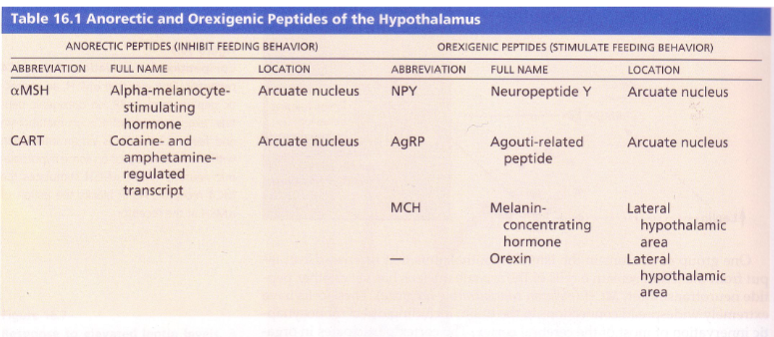
What are orexinogenic peptides and which ones increase appetite? (1+5)
Orexinogenic peptides are endogenous neuropeptides that stimulate appetite.
Examples include:
Ghrelin
Orexin
Agouti-related peptide (AgRP)
Melanin-concentrating hormone (MCH)
Neuropeptide Y (NPY)
What are anorectic peptides and which ones suppress appetite? (1+2)
Anorectic peptides are endogenous neuropeptides that suppress appetite.
Examples include:
αMSH (Alpha-Melanocyte Stimulating Hormone)
CART (Cocaine and Amphetamine Regulated Transcript)