Second law of thermodynamics from a systems view
The second law of thermodynamics states that objects in thermal contact and not in thermal equilibrium will exchange heat energy such that the object with a higher temperature will give off heat energy to the object with a lower temperature until both objects have the same temperature at thermal equilibrium. As such, energy is constantly being dispersed.
Second law of thermodynamics from an entropy view
The second law of thermodynamics states that energy spontaneously disperses from being localized to becoming spread out if it is not hindered from doing so.
Do NOT think of entropy as _____!
disorder
Entropy
Entropy is the measure of the spontaneous dispersal of energy at a specific temperature: how much energy is spread out, or how widely spread out energy becomes in a process.
Which of these systems has a higher entropy: 1 gram of water at 0°C, or 1 gram of ice at 0°C? Why?
Water
Water has more degrees of freedom, so essentially the kinetic energy is dispersed over a larger number of microstates. In this sense, water is less organized, or more disordered, than ice.
Change in Entropy equation
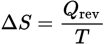
ΔS is the change in entropy
Qrev is the heat that is gained or lost in a reversible process
T is the temperature in kelvin
Units of entropy
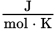
When energy is distributed into a system at a given temperature, its entropy _____. When energy is distributed out of a system at a given temperature, its entropy _____.
increases
decreases
Notice that the second law states that energy will spontaneously disperse; it does not say that energy can never be _____. However, the concentration of energy will not _____.
localized or concentrated
happen spontaneously in a closed system
_____ usually must be done to concentrate energy.
work
Remember that a system can be variably defined to include the entire universe; in fact, the second law ultimately claims that _____.
the entropy of the universe is increasing
Change in entropy of the universe equation
ΔSuniverse = ΔSsystem + ΔSsurroundings > 0
Reversible reaction
A hypothetical process whereby an infinitesimally small amount of heat is transferred from a system to its surroundings. Because the heat transfers are infinitesimally small, the system is always in equilibrium with the surroundings.
How long would a reversible reaction take place over?
An infinite amount of time (thus making it impossible)
What is the change in entropy of a reversible process?
ΔSuniverse = 0
What is an example of a reversible process?
If we place a mixture of ice and liquid water into a thermostat that is also at 0°C and allow infinitesimal amounts of heat to be absorbed by the ice from the thermostat so that the ice melts to liquid water at 0°C and the thermostat remains at 0°C, then the increase in the entropy of the system (the water) will be exactly equal to the entropy decrease of the surroundings (the thermostat).
What is the difference between a reversible process in physics and one in chemistry (or anything else for that matter)?
In physics, a reversible process is one which causes no net change in entropy of the universe. Freezing and unfreezing ice is clearly a reversible process on the chemical level, by by doing this over a reasonable time span the entropy of the universe increases, making it physically irreversible.