First law of thermodynamics
The first law of thermodynamics states that the change in the total internal energy of a system is equal to the amount of energy transferred in the form of heat to the system, minus the amount of energy transferred from the system in the form of work.
Change in Internal Energy equation
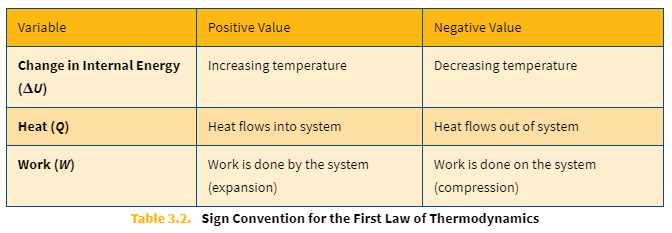
ΔU = Q − W
ΔU = change in internal energy
Q = heat
W = work
Sign conventions of the First Law of Thermodynamics
Law of energy conservation
Energy can be neither created nor destroyed; it can only be changed from one form to another.
The first law is really just a particular iteration of the more universal physical _____.
law of energy conservation
Second law of thermodynamics
Objects in thermal contact and not in thermal equilibrium will exchange heat energy such that the object with a higher temperature will give off heat energy to the object with a lower temperature until both objects have the same temperature at thermal equilibrium
Heat
Heat is defined as the process by which a quantity of energy is transferred between two objects as a result of a difference in temperature.
Heat can never spontaneously transfer energy from a cooler object to a warmer one without ...
... work being done on the system.
SI unit for heat
joule (J)
Alternative units of heat
calorie (cal), nutritional Calorie (Cal), British thermal unit (BTU)
Conversion between calories and nutritional Calories
1 nutritional Calorie ("big C") = 1000 calories ("little c")
Conversion between the four common units of heat
1 Cal ≡ 103 cal = 4184 J = 3.97 BTU
One calorie (little c) is the amount of heat required to ...
... raise 1 g of water one degree Celsius.
One Calorie (big C) is the amount of heat required to ...
... raise 1 kg of water 1 degree Celsius, equal to 1000 calories.
There are three means by which heat can transfer energy: _____, _____, and _____.
conduction, convection, and radiation
Conduction
Conduction is the direct transfer of energy from molecule to molecule through molecular collisions.
Metals are described as the best heat conductors because ...
... metallic bonds contain a density of atoms embedded in a sea of electrons, which facilitate rapid energy transfer.
Gases tend to be the poorest heat conductors because ...
... there is so much space between individual molecules that energy-transferring collisions occur relatively infrequently.
Conduction requires ...
... direct physical contact between the objects.
Convection
Convection is the transfer of heat by the physical motion of a fluid over a material.
Because convection involves flow, ...
... only liquids and gases can transfer heat by this means.
Radiation
Radiation is the transfer of energy by electromagnetic waves.
Unlike conduction and convection, ...
... radiation can transfer energy through a vacuum.
Specific heat (with abbreviation and units)
The specific heat (c) of a substance is defined as the amount of heat energy required to raise one gram of a substance by one degree Celsius or one unit kelvin.
The specific heat of liquid water is _____ (J)
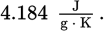
Formula relating heat gained/lost to change in temperature
q = mcΔT
q = heat gained/lost
m = mass
c = specific heat of the substance
ΔT = change in temperature
The specific heat of liquid water is _____ (cal)
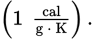
Mnemonic: The equation for heat transfer, given a specific heat, is the same as ...
... the test you’re studying for!
q = mcΔT looks a lot like “q equals MCAT.”
When a substance is undergoing a phase change, the heat that is added or removed from the system ...
... does not result in a change in temperature.
Phase changes occur at a constant temperature, and the temperature will not begin to change until ...
... all of the substance has been converted from one phase into the other.
Phase changes are related to changes in _____, not _____.
potential energy
kinetic energy
When a phase change occurs, the increase in potential energy is due to ...
... an increase in the degrees of freedom of movement.
Alternatively, one may think of this as an increase in the potential microstates of the molecules.
The average kinetic energy of liquid water at 0°C and solid water at 0°C is _____.
the same
Phase change equation
q = mL
q = amount of heat gained or lost
m = mass
L = heat of transformation, or latent heat
Phase changes from liquid to solid
freezing or solidification
Phase changes from solid to liquid
melting or fusion
Melting point
The temperature at which the solid/liquid phase change occurs.
Heat of fusion
The heat of transformation that occurs at the melting point
Phase change from liquid to gas
boiling, evaporation, or vaporization
Phase change from gas to liquid
condensation
Boiling point
The temperature at which the liquid/gas phase change occurs.
Heat of vaporization
The heat of transformation that occurs at the boiling point.
Phase changes from solid to gas
sublimation
Phase changes from gas to solid
deposition
Work accomplished by a change in displacement is not likely to be motivated by _____.
heat transfer
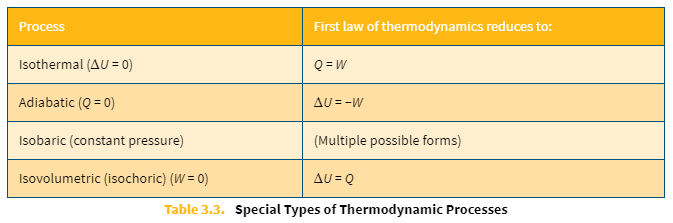
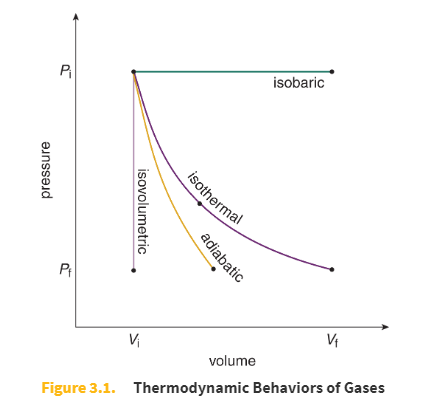
Special types of thermodynamic processes table
Isothermal process equations
ΔU = 0
Q = W
Adiabatic process equations
Q = 0
ΔU = −W
Isobaric (constant pressure) process equations
multiple
Isovolumetric (isochoric) process equations
W = 0
CU = Q
Isothermal process
Constant temperature, and therefore no change in internal energy
Adiabatic process
No heat exchange
Isovolumetric (also called Isochoric) process
No change in volume, and therefore no work accomplished
Isobaric process
Constant pressure
Thermodynamic Behaviors of Gases diagram (pressure vs volume)
Isobaric work equation
W = PΔV
The specific heat of liquids is generally _____ than that of solids. Why?
higher
Liquids have higher degrees of freedom than solids, so there are more ways for liquids to absorb heat w/o experiencing the same change in temperature (far from a rigorous answer)