The zeroth law of thermodynamics is based on a simple observation: when one object is in thermal equilibrium with another object, and the second object is in thermal equilibrium with a third object, then ...
... the first and third object are also in thermal equilibrium.
Note that thermal contact does not necessarily imply physical contact, as objects can be in thermal contact across space.
The zeroth law of thermodynamics states the transitive property in thermal systems: If a = band b = c, then _____.
a = c
Formulation of the zeroth law
-No net heat flows between objects in thermal equilibrium
-Corollary: heat flows between two objects not in thermal equilibrium
Relevant points in the three temperature scales
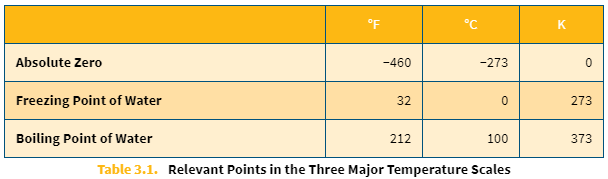
Conversion between the three common temperature scales
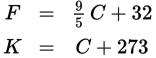
What are the three common temperature scales?
Fahrenheit (°F), Celsius (°C), and Kelvin (K)
Absolute zero
The theoretical temperature at which there is no thermal energy
What temperature scale is most commonly used in science, and why?
Kelvin, because it is an absolute temperature scale (zero reference point set to absolute zero)
Third law of thermodynamics
The entropy of a perfectly organized crystal at absolute zero is zero.
The only time Fahrenheit is used routinely on the MCAT is for body temperature, which is ...
... 98.6°F or 37°C.
Length, volume, solubility, and even the conductivity of matter change as a function of _____.
temperature
Thermal expansion
The tendency of matter to change its shape, area, volume, and density in response to a change in temperature.
Length thermal expansion mnemonic
When the temperature of an object changes, its length changes a lot (αLΔT).
Thermal expansion equation in one dimension
ΔL = αLΔT
ΔL = change in length
α = coefficient of linear expansion
L = length
ΔT = change in temperature
Coefficient of linear expansion (with units)
A constant that characterizes how a specific material’s length changes as the temperature changes.
This usually has units of K–1, although it may sometimes be quoted as °C–1.
Volumetric thermal expansion equation for liquids and solids
ΔV = βVΔT
ΔV = change in volume
β = coefficient of volumetric expansion
V = volume
ΔT = change in temperature
Coefficient of volumetric expansion (with calculation)
A constant that characterizes how a specific material’s volume changes as the temperature changes.
Its value is equal to three times the coefficient of linear expansion for the same material (β = 3α).