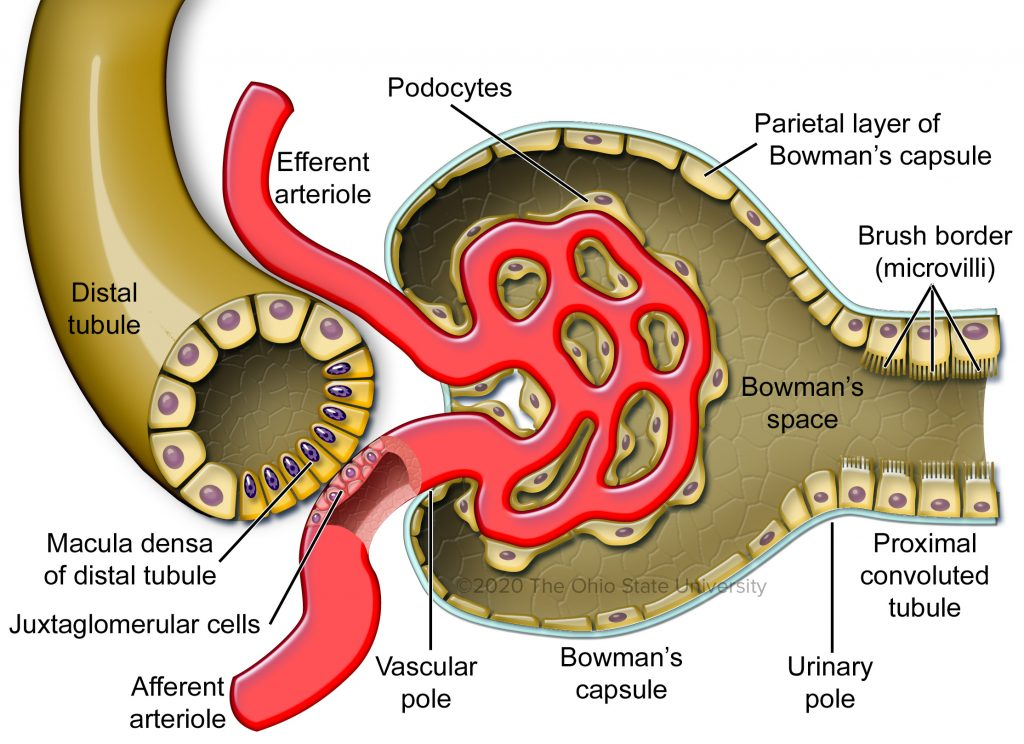
What structures make up the renal corpuscle?
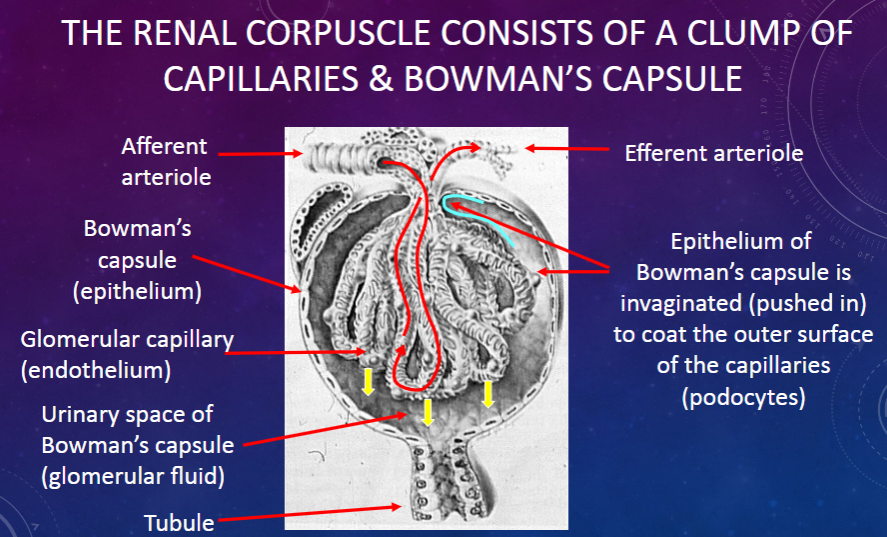
𖹭 A clump of capillaries and Bowman’s capsule: The renal corpuscle consists of these components.
What is the function of the epithelium of Bowman’s capsule?
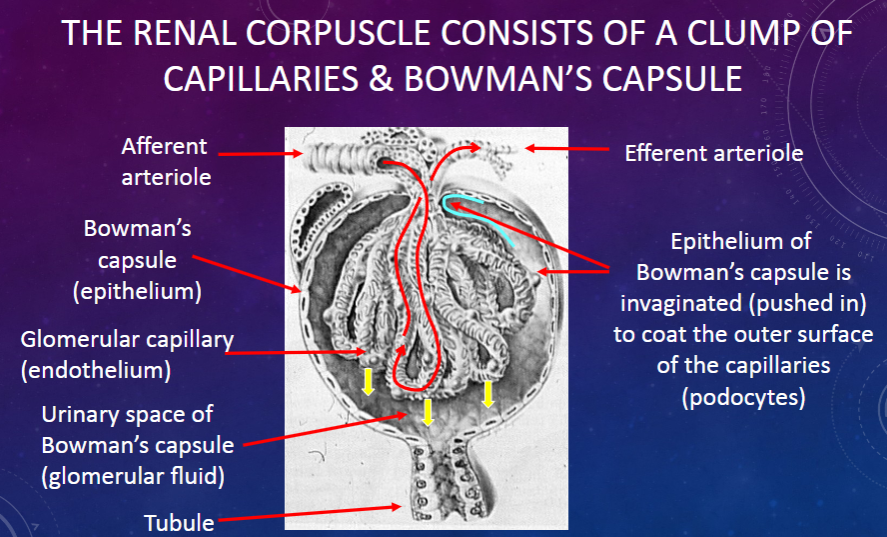
𖹭 To coat the outer surface of the capillaries (podocytes): The epithelium of Bowman’s capsule is invaginated to cover the capillaries.
What are the roles of the afferent and efferent arterioles in the renal corpuscle?
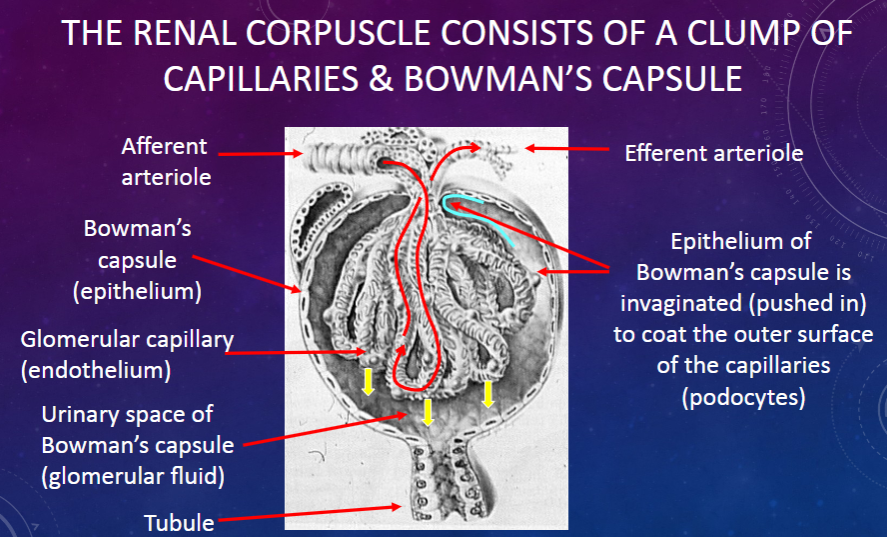
𖹭 Afferent arteriole: Supplies blood to the glomerulus.
𖹭 Efferent arteriole: Carries blood away from the glomerulus.
What is the urinary space in Bowman’s capsule?
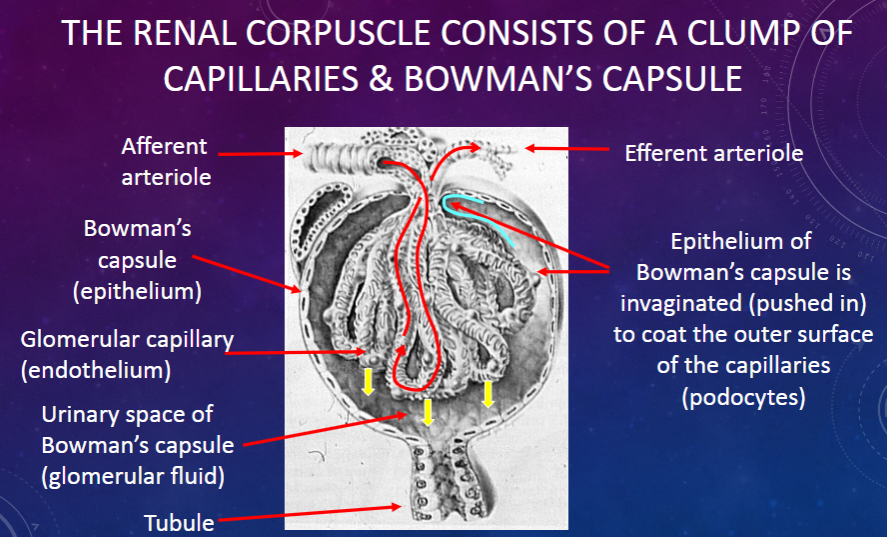
𖹭 The space where the filtrate collects: It is the area between the visceral and parietal layers of Bowman’s capsule.
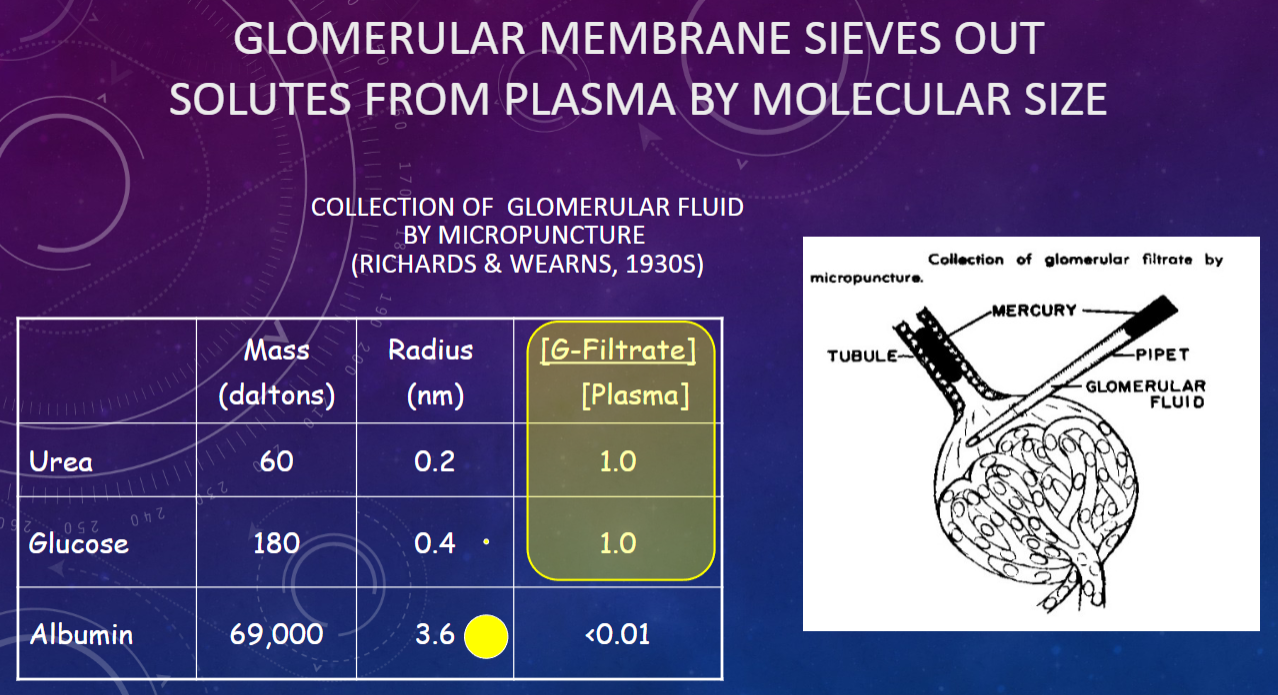
What did Ludwig realize about glomerular fluid in 1844?
𖹭 It is a passive ultrafiltrate of plasma: Plasma proteins are filtered out, resulting in glomerular fluid.
What are the concentrations of small solutes like NaCl, glucose, and urea in glomerular fluid compared to plasma?
𖹭 Concentration in glomerular fluid = concentration in plasma: Small solutes pass through the filtration barrier.
What is the concentration of plasma proteins in glomerular fluid?
𖹭 Concentration in glomerular fluid = almost zero: Plasma proteins are largely excluded from the filtrate.
Why is urine routinely tested for protein (proteinuria) in clinical settings?
𖹭 Proteinuria is a sign of renal/urinary tract disease: Presence of protein in urine indicates a potential problem with kidney function.
What drives the ultrafiltration process across the glomerular membrane?
𖹭 A net pressure drop across the glomerular membrane: This pressure differential facilitates the filtration of plasma into the glomerular fluid.
Picture demonstrating Glomerular membrane sieves out solutes from by molecular size:
What drives glomerular fluid formation (filtration)?
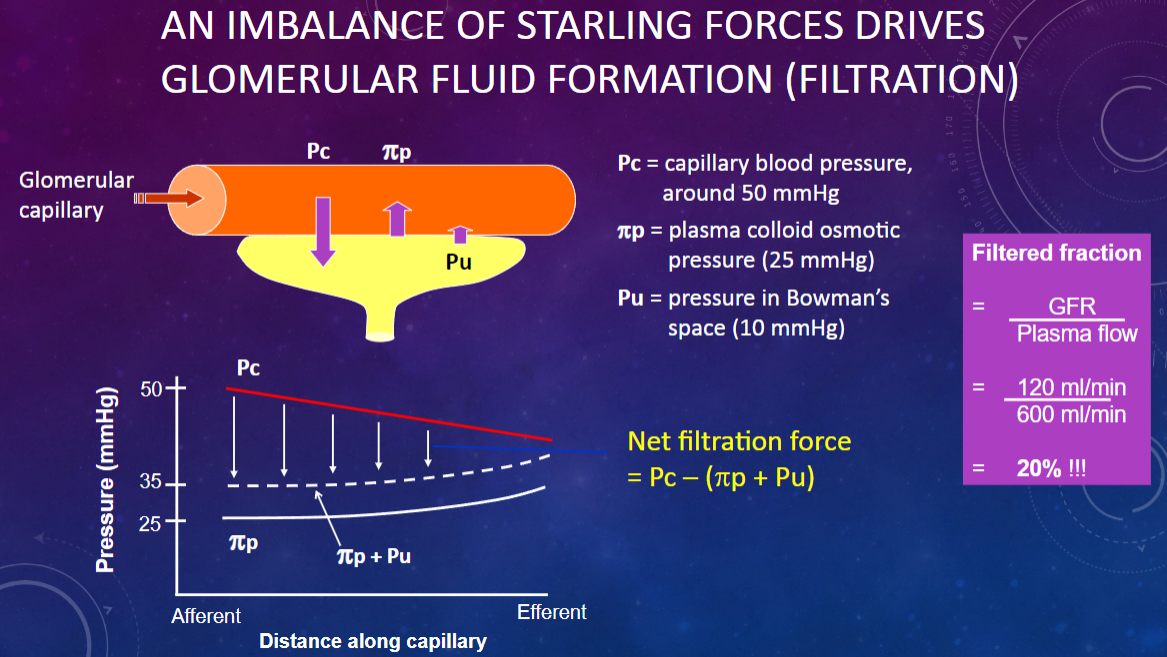
𖹭 An imbalance of Starling forces: This imbalance is crucial for the filtration process.
What is the capillary blood pressure (Pc) in the glomerulus?
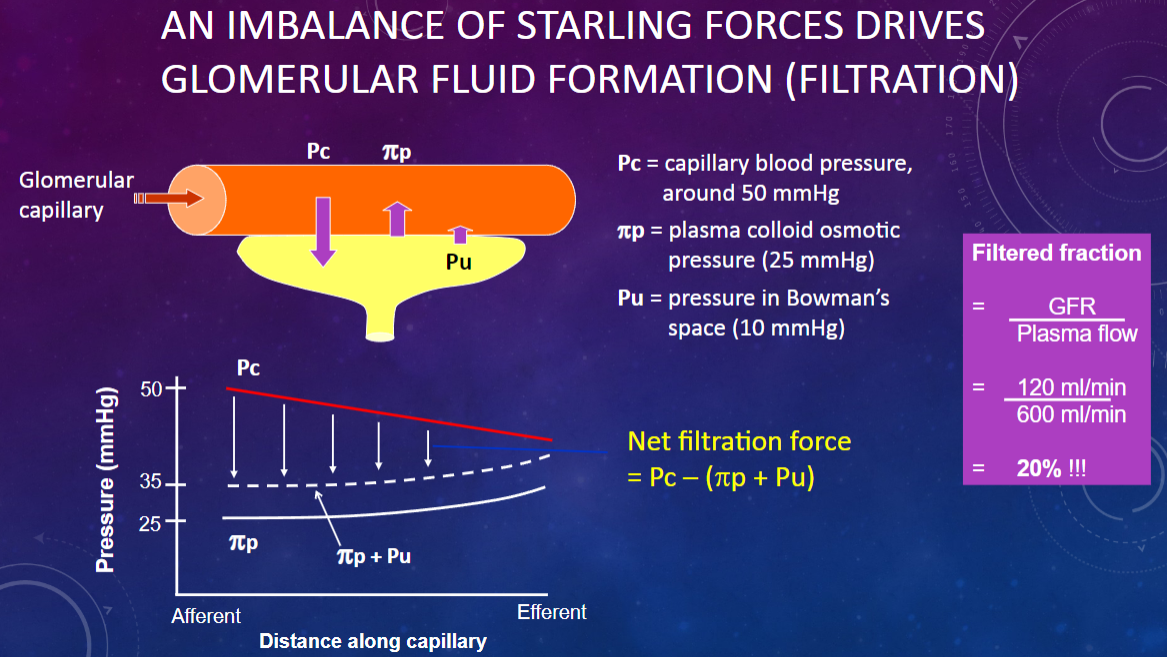
𖹭 Around 50 mmHg: This pressure pushes fluid out of the capillaries.
What is the plasma colloid osmotic pressure (πp) in the glomerulus?
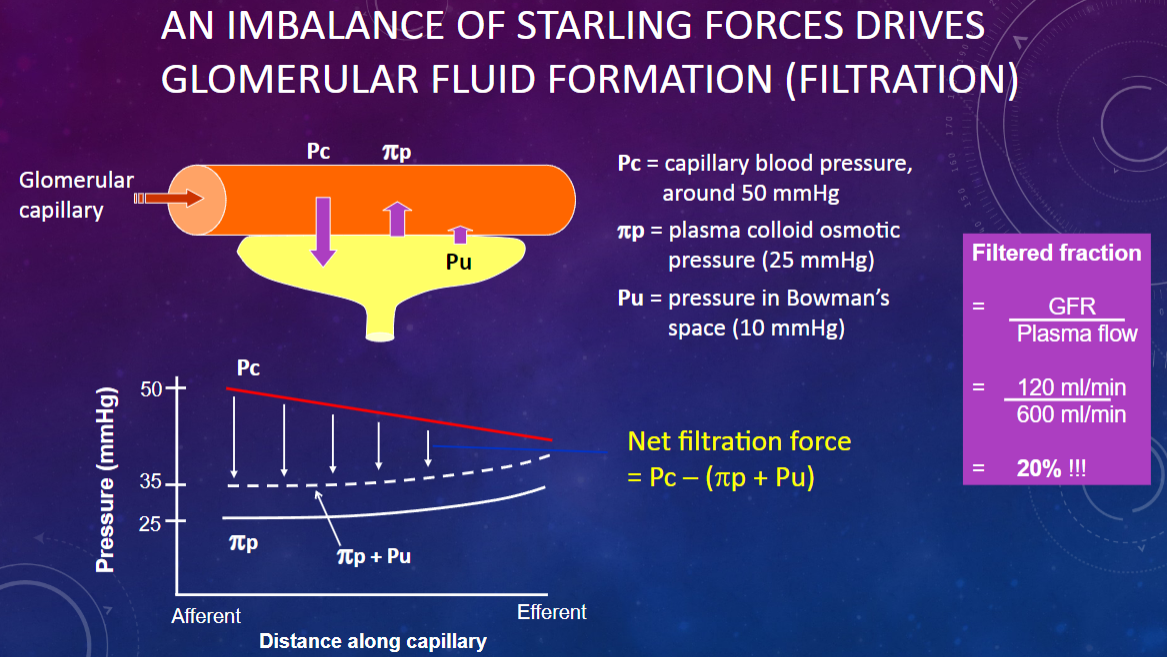
𖹭 25 mmHg: This pressure opposes filtration by drawing fluid back into the capillaries.
What is the pressure in Bowman’s space (Pu)?
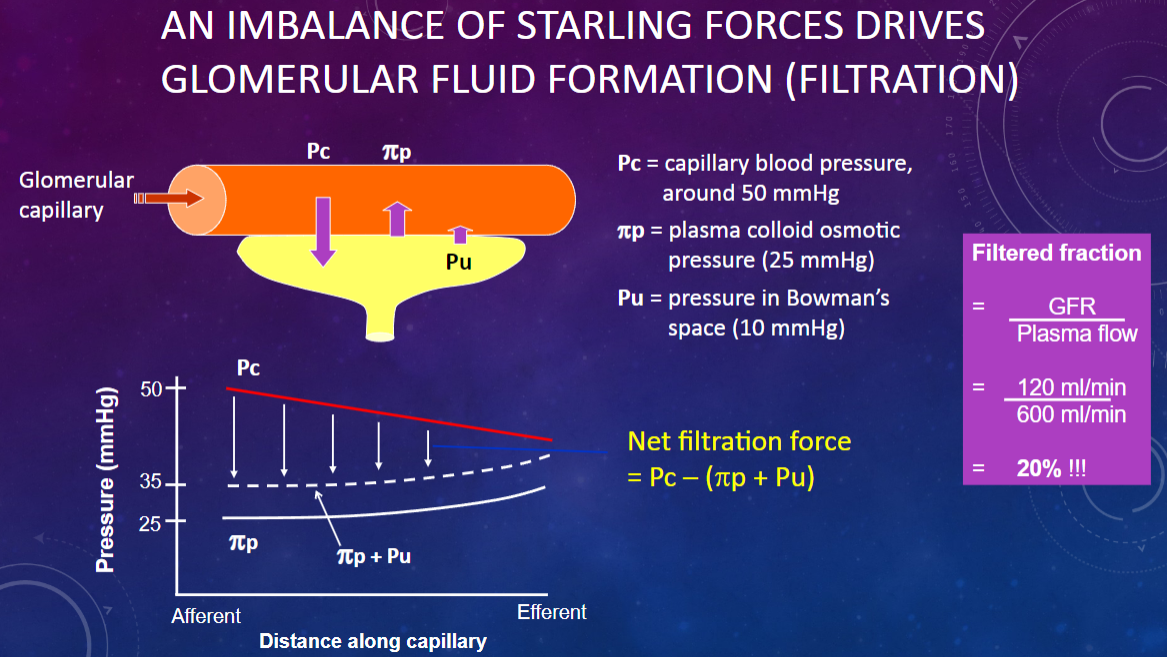
𖹭 10 mmHg: This pressure also opposes filtration by pushing fluid back into the capillaries.
How do the Starling forces balance in glomerular filtration?
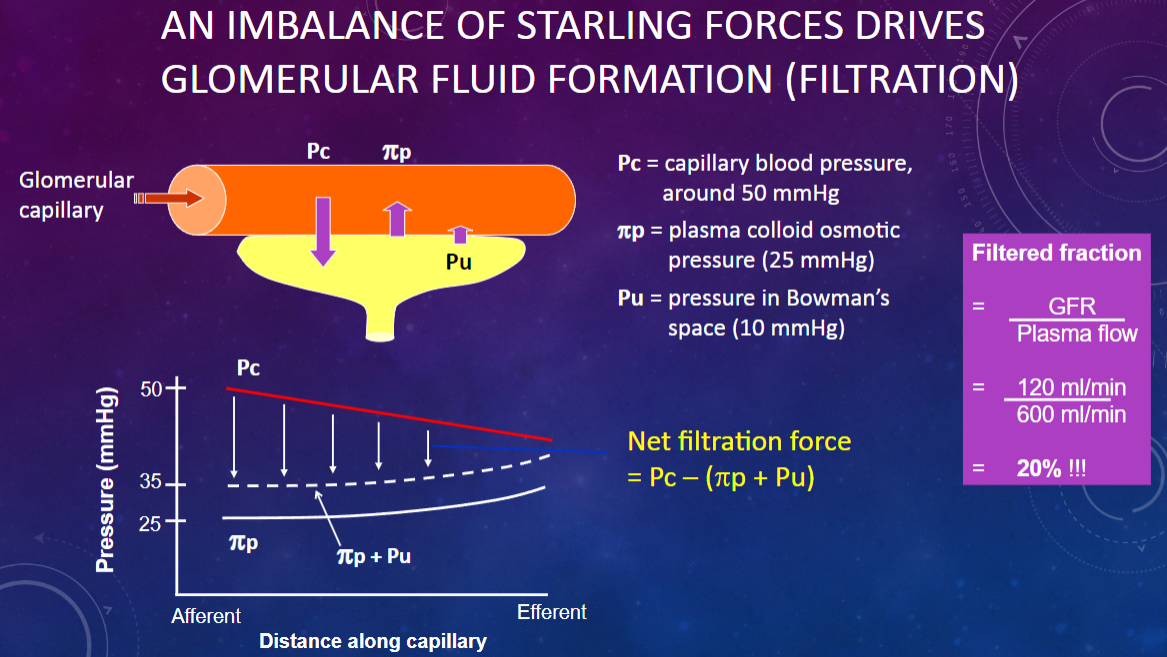
𖹭 Capillary blood pressure (50 mmHg) pushes fluid out, while plasma colloid osmotic pressure (25 mmHg) and Bowman’s space pressure (10 mmHg) oppose this, leading to a net filtration pressure.
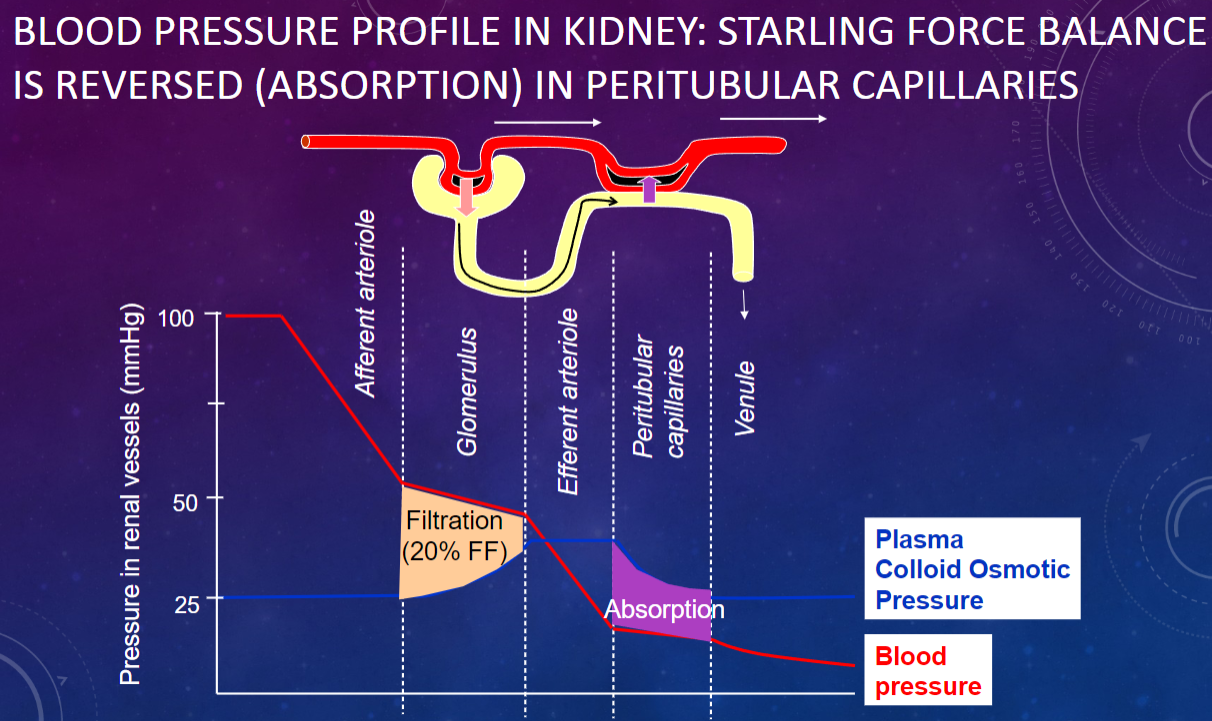
Picture demonstrating BLOOD PRESSURE PROFILE IN KIDNEY: STARLING FORCE BALANCE IS REVERSED (ABSORPTION) IN PERITUBULAR CAPILLARIES:
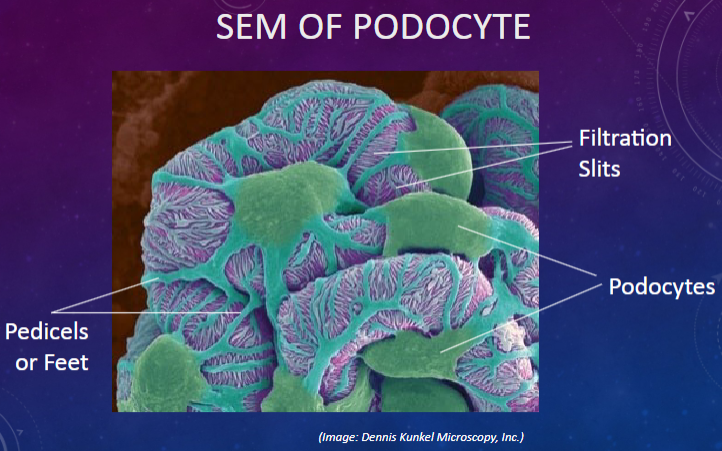
Picture demonstrating SEM OF PODOCYTE:
What is the structure of the glomerular membrane? (3)
𖹭 The glomerular membrane consists of:
-Podocyte foot processes (pedicels)
-Basal lamina (lamina rara, lamina densa, lamina rara)
-Fenestrated endothelium of the capillary
What is the function of podocytes in the glomerulus?
𖹭 Podocytes form the epithelial layer of Bowman’s capsule and create filtration slits with their foot processes.
What are filtration slits?
𖹭 Gaps between the foot processes of podocytes that allow for filtration of small molecules.
What are the components of the basal lamina in the glomerulus?
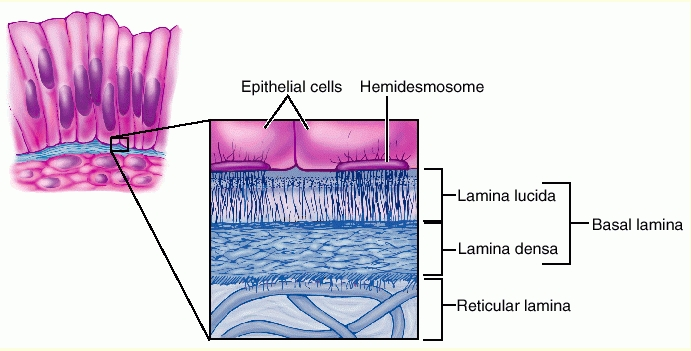
The basal lamina includes:
Lamina rara
Lamina densa
Lamina rara (repeated)
What type of endothelium is found in the glomerular capillaries?
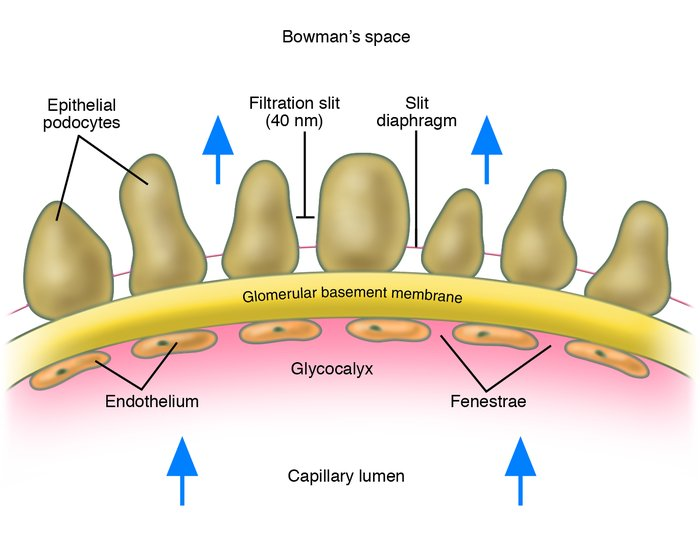
𖹭 Fenestrated endothelium, which contains pores to facilitate filtration.
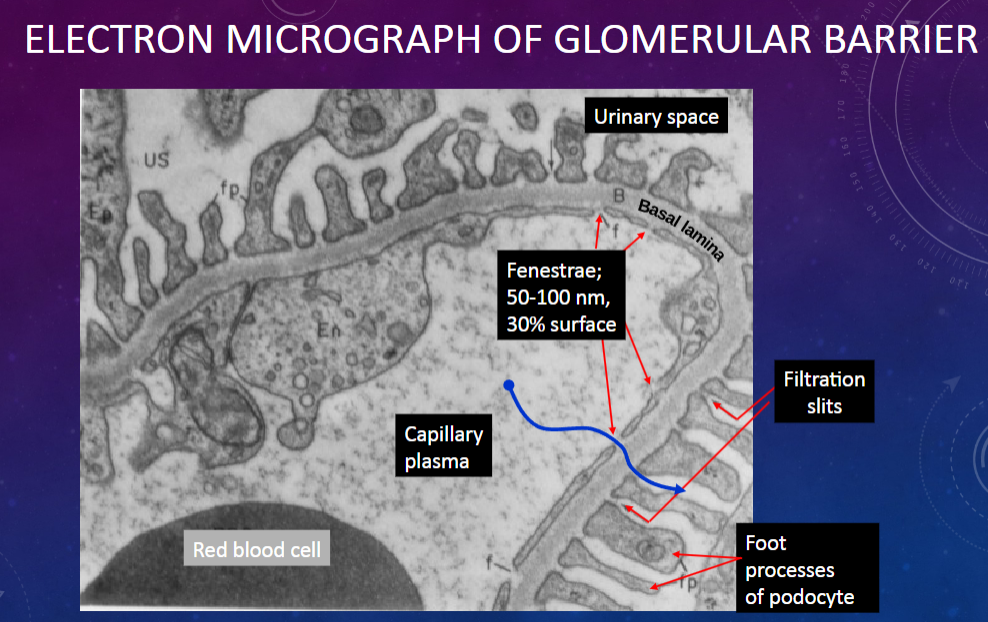
Picture demonstrating ELECTRON MICROGRAPH OF GLOMERULAR BARRIER:
Picture demonstrating SURFACE VIEW OF FILTRATION SLITS BETWEEN FOOT PROCESSES, SEEN FROM URINARY SPACE:
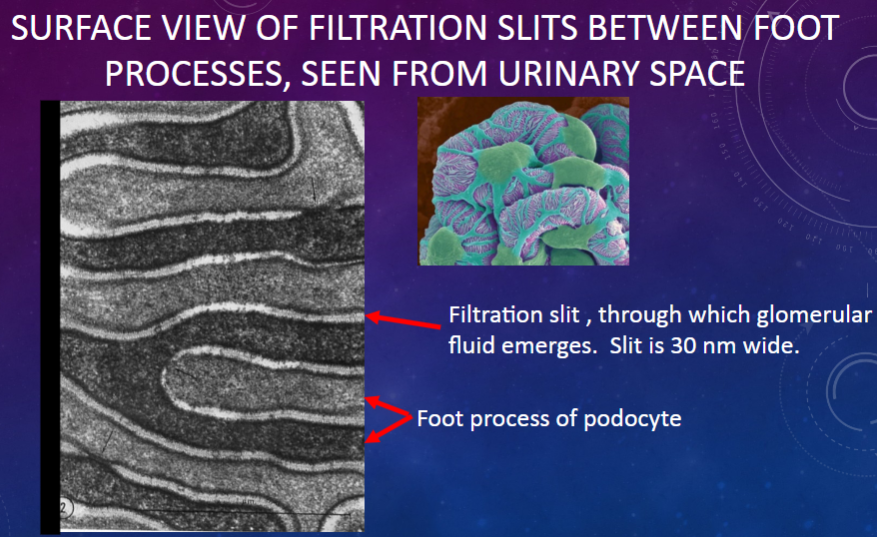
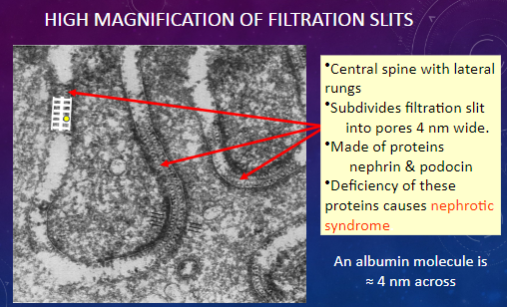
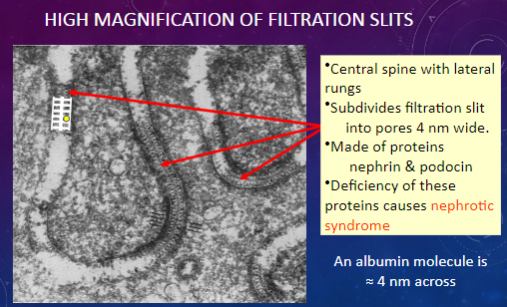
What structures are observed in a high magnification view of filtration slits?
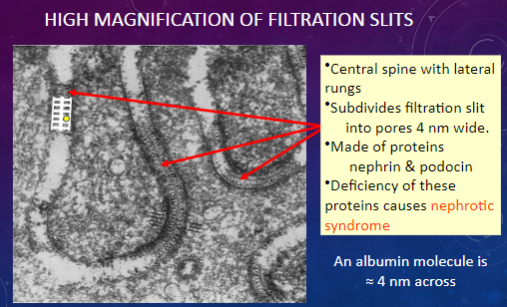
𖹭 Central spine with lateral rungs: Subdivides the filtration slit into smaller pores.
𖹭 Pores: Approximately 4 nm wide.
𖹭 Made of proteins nephrin & podocin.
What is the approximate width of the pores in filtration slits?
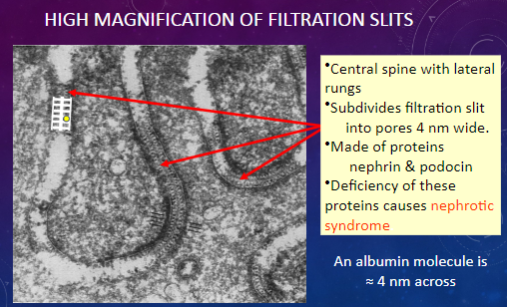
𖹭 4 nm: These small pores allow for the passage of molecules such as albumin.
Which proteins are involved in forming the central spine and lateral rungs of filtration slits?
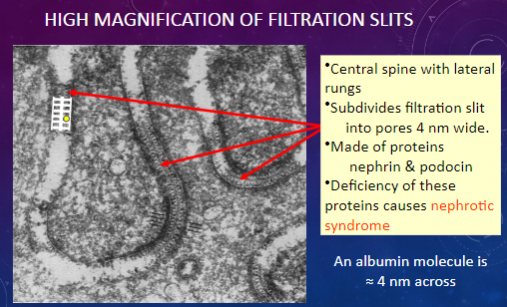
𖹭 Nephrin & podocin: Deficiency of these proteins can lead to nephrotic syndrome.
What is the significance of the width of filtration slit pores compared to the size of an albumin molecule?
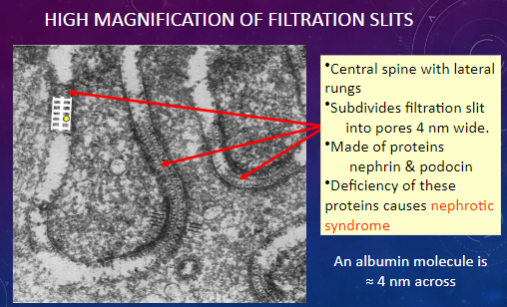
𖹭 An albumin molecule is approximately 4 nm across, which is similar in size to the pores in filtration slits.
What condition can result from a deficiency of nephrin and podocin proteins in the filtration slits?
𖹭 Nephrotic syndrome: A disorder characterized by proteinuria, hypoalbuminemia, and edema.
Which layer in the glomerular membrane acts as a molecular sieve?
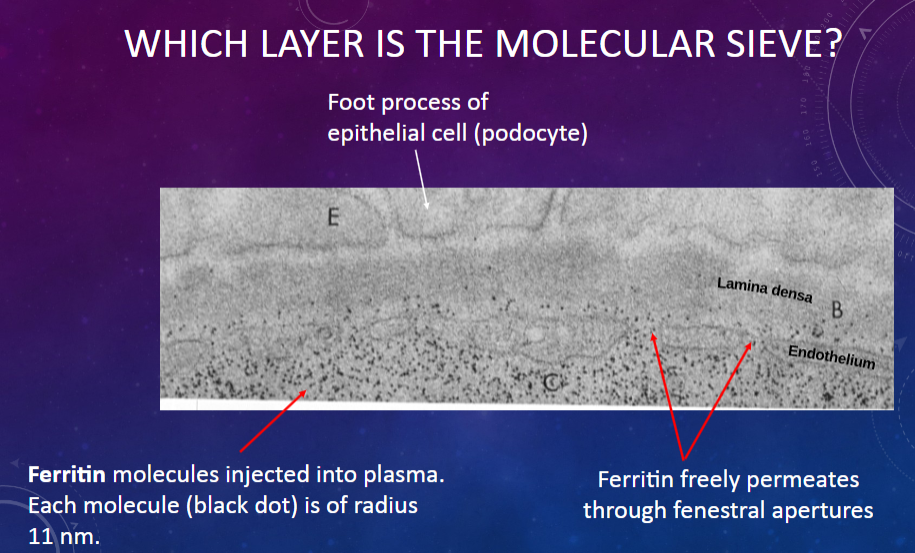
𖹭 The fenestrated endothelium of the glomerular capillary: It allows the passage of molecules based on their size.
What is the size of ferritin molecules injected into plasma?
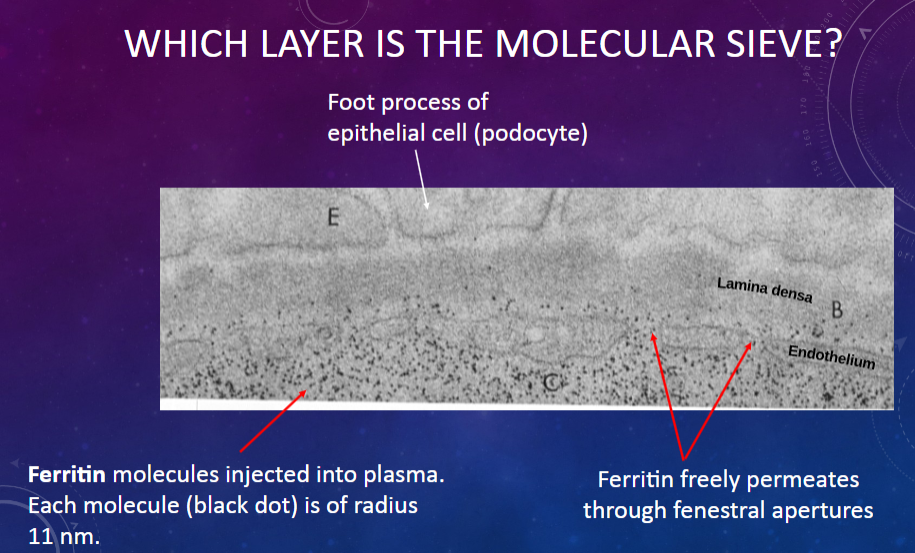
𖹭 Each molecule has a radius of 11 nm.
What is the permeability of the glomerular membrane to ferritin molecules?
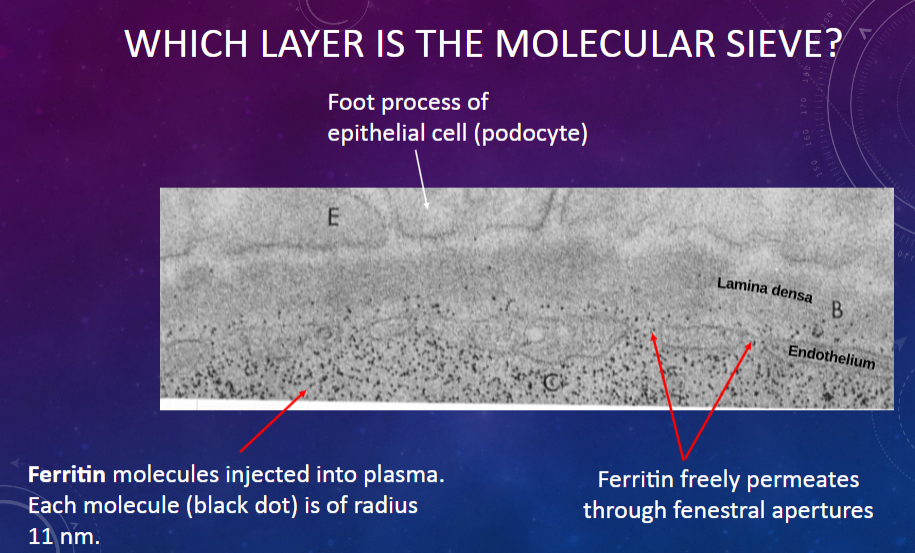
𖹭 Ferritin freely permeates through the fenestral apertures of the endothelium.
What allows ferritin molecules to pass through the glomerular membrane?
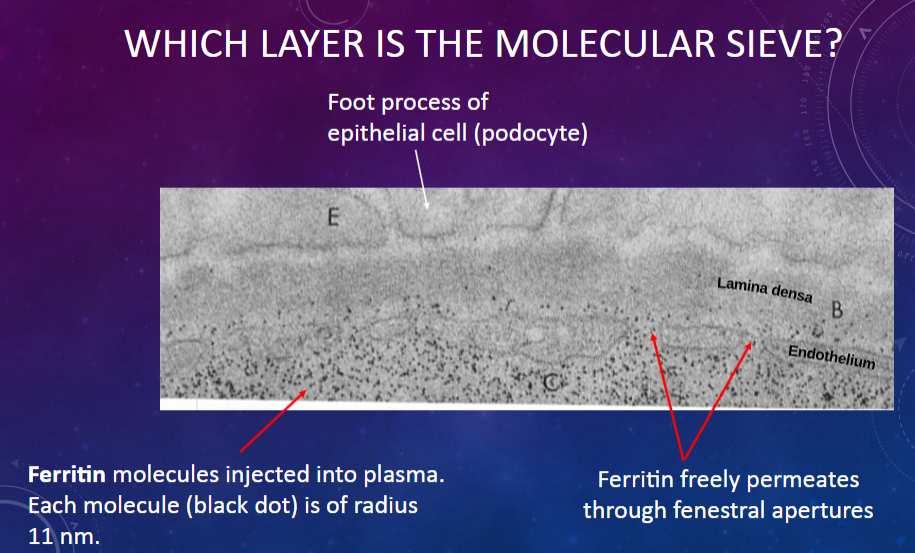
𖹭 The fenestral apertures in the endothelium, which act as pores for molecular passage.
Which cell type in the glomerular membrane forms foot processes that contribute to the filtration barrier?
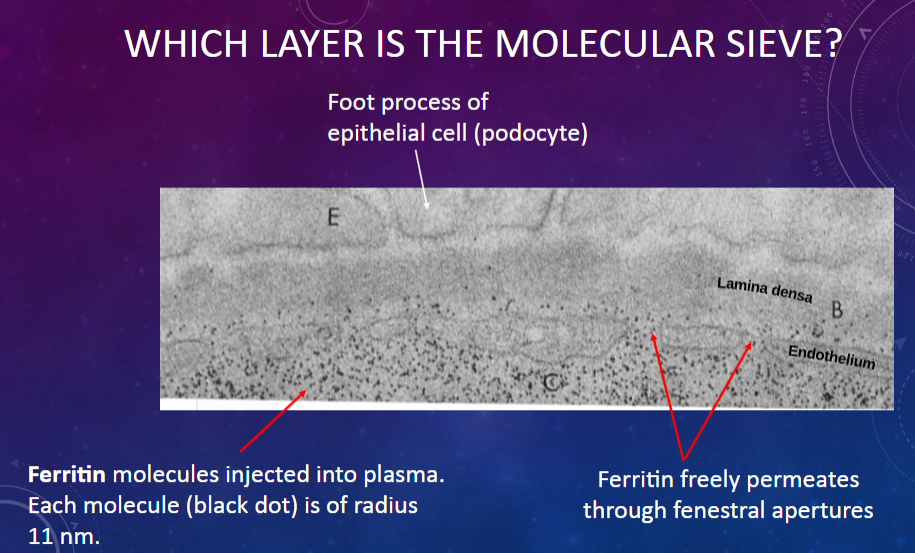
𖹭 Epithelial cells or podocytes: Their foot processes create filtration slits through which molecules pass.
What happens to myeloperoxidase molecules injected into plasma as they encounter the glomerular filtration barrier?
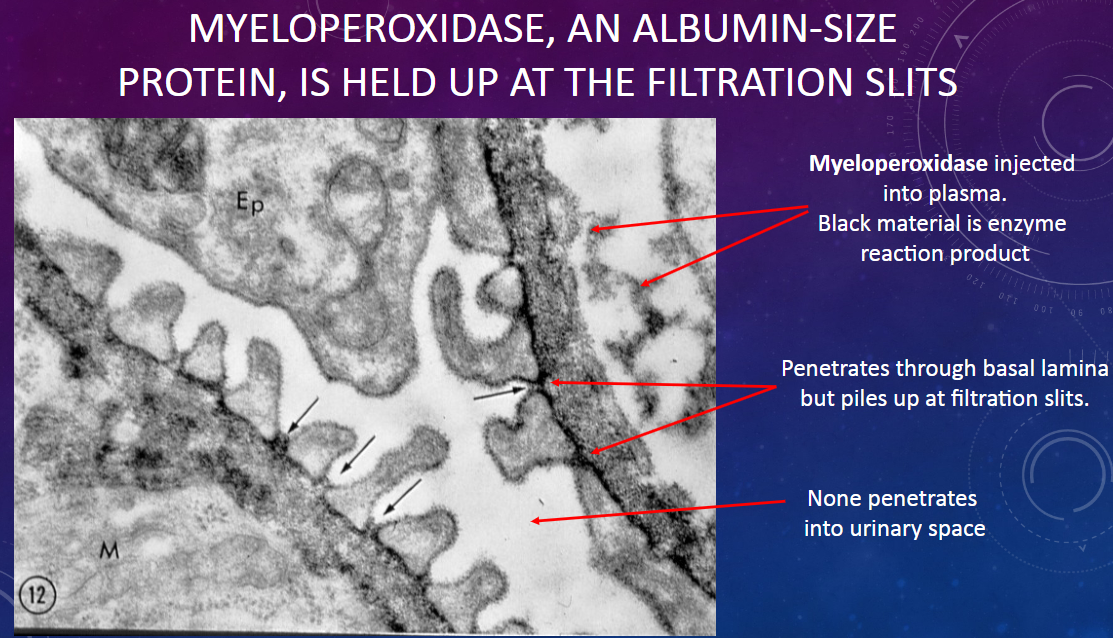
𖹭 Myeloperoxidase penetrates through the basal lamina but accumulates at the filtration slits.
What is the size of myeloperoxidase molecules compared to albumin?
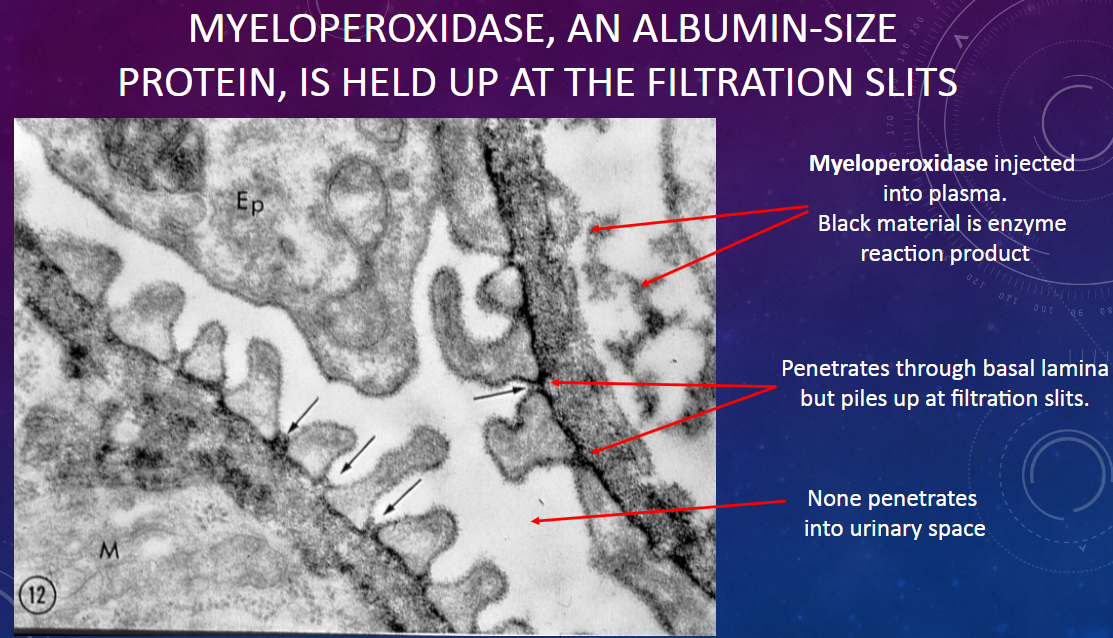
𖹭 Myeloperoxidase is approximately the same size as albumin molecules.
What is observed regarding the passage of myeloperoxidase through the glomerular membrane?
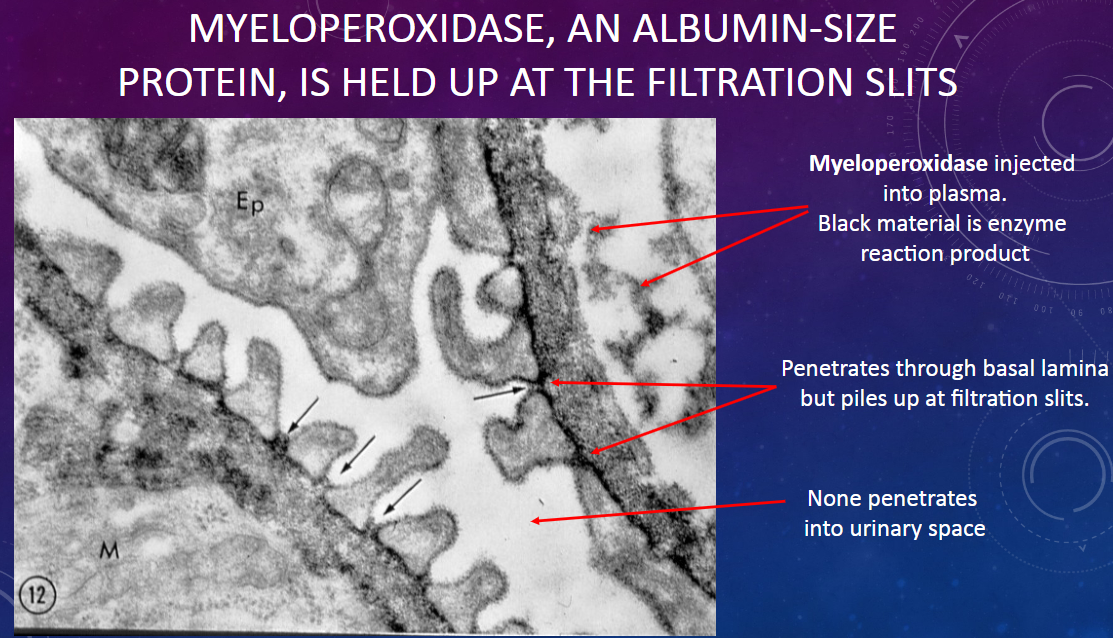
𖹭 None of the myeloperoxidase penetrates into the urinary space; it accumulates at the filtration slits.
Where does myeloperoxidase accumulate within the glomerular filtration barrier?
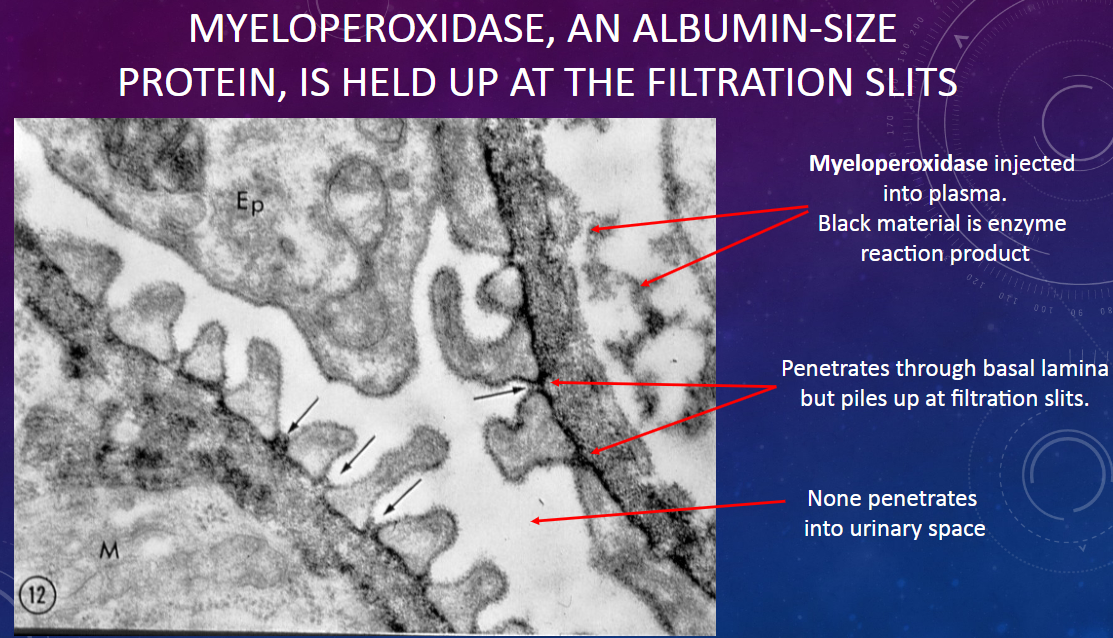
𖹭 Myeloperoxidase accumulates specifically at the filtration slits, without penetrating into the urinary space.
What does the behavior of myeloperoxidase at the filtration slits suggest about its permeability through the glomerular membrane?
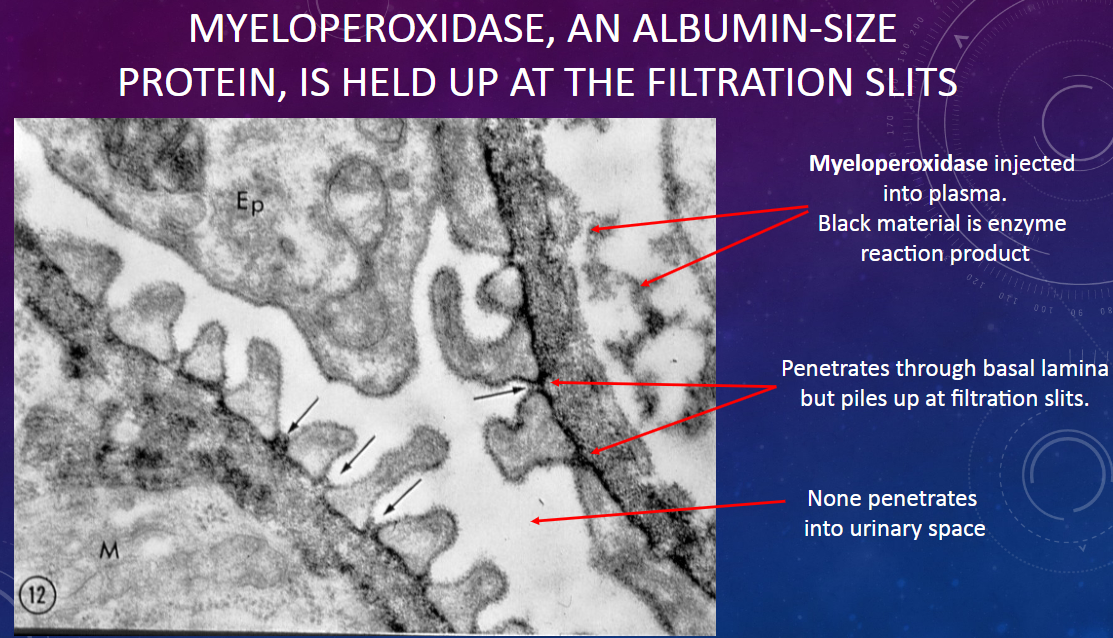
𖹭 It suggests that myeloperoxidase is held up at the filtration slits, indicating a selective barrier to its passage.
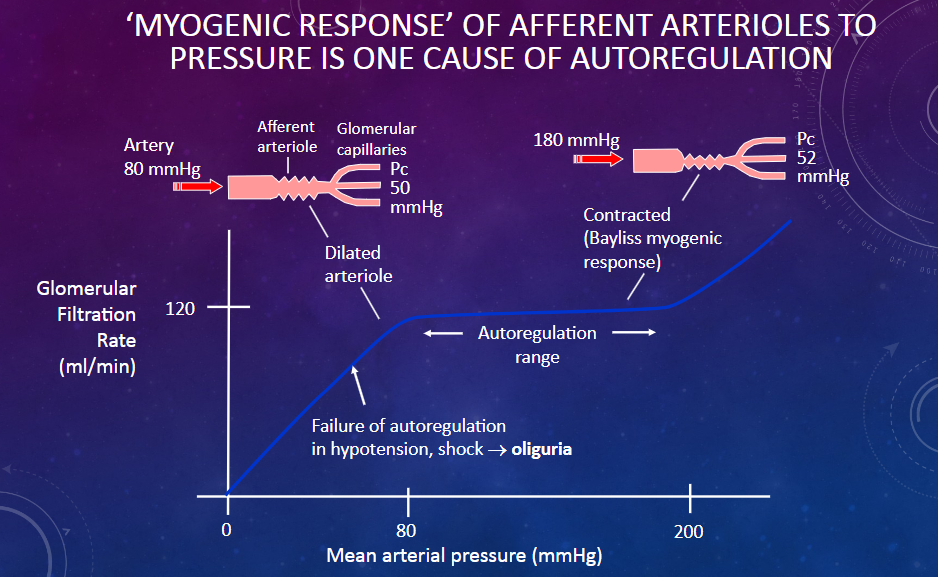
What happens if glomerular blood pressure is too low?
𖹭 Low glomerular blood pressure may lead to decreased glomerular filtration rate (GFR) and reduced urine formation.
What happens if glomerular blood pressure is too high?
𖹭 High glomerular blood pressure can cause damage to the glomerular capillaries and lead to increased glomerular filtration rate (GFR), potentially causing kidney damage over time.
How is pressure within the glomerular capillaries regulated?
𖹭 Pressure within the glomerular capillaries is regulated through autoregulation mechanisms within the kidney and extrinsic mechanisms involving the peripheral nervous system (PNS).
What are the components of autoregulation mechanisms for controlling pressure within the glomerular capillaries?
𖹭 Autoregulation mechanisms include the Bayliss myogenic response and tubuloglomerular feedback, both of which operate within the kidney.
How does the Bayliss myogenic response contribute to pressure regulation within the glomerular capillaries?
𖹭 The Bayliss myogenic response is a mechanism within the kidney that helps to regulate glomerular blood pressure by adjusting the diameter of the afferent arteriole in response to changes in blood pressure.
What is the role of tubuloglomerular feedback in controlling pressure within the glomerular capillaries?
𖹭 Tubuloglomerular feedback is a mechanism that involves communication between the macula densa cells of the distal tubule and the afferent arteriole to regulate glomerular blood pressure based on changes in tubular fluid composition.
What is the Bayliss myogenic response?
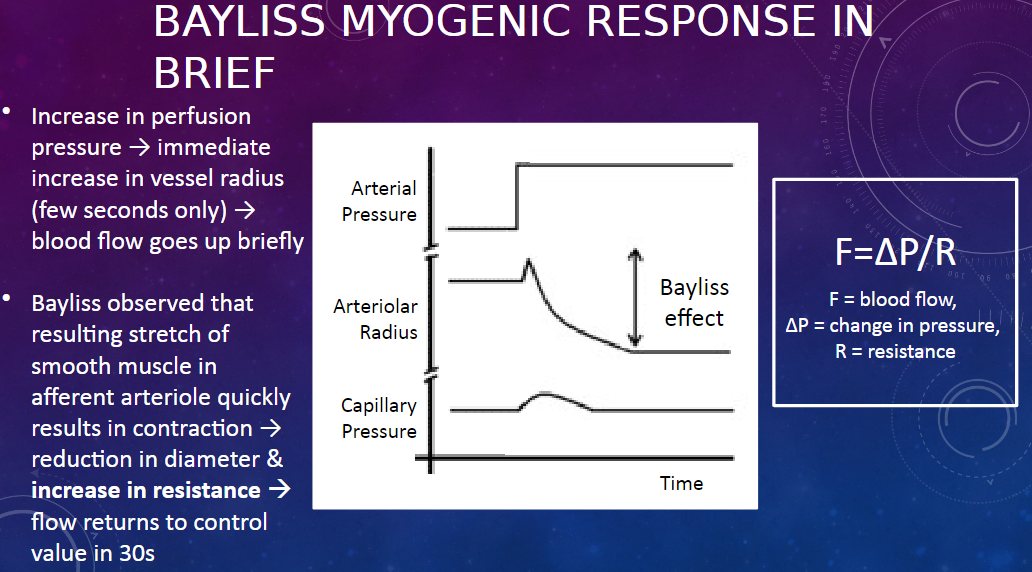
𖹭 The Bayliss myogenic response is a mechanism that regulates renal blood flow in response to changes in perfusion pressure.
How does the Bayliss myogenic response work?
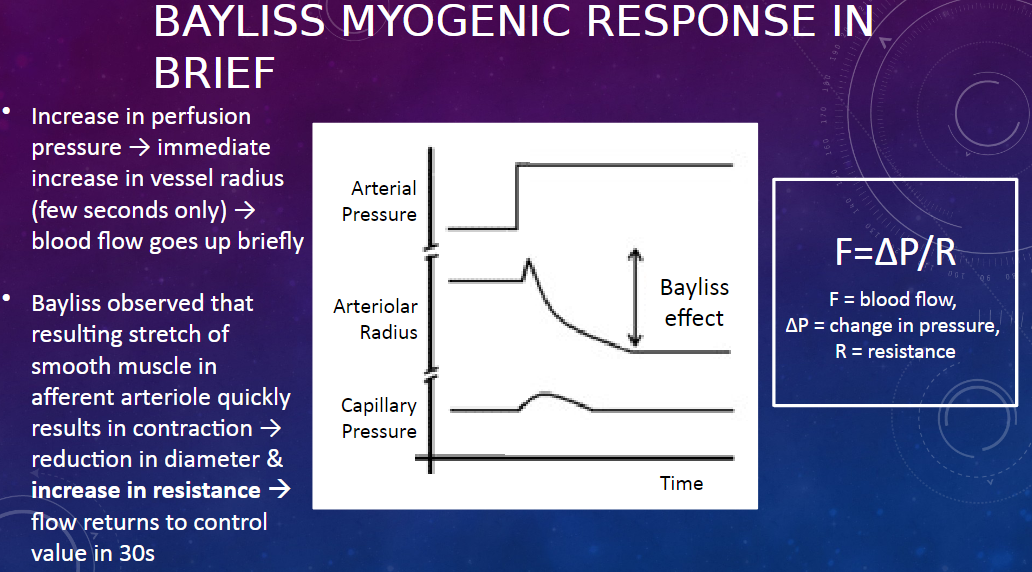
𖹭 When there is an increase in perfusion pressure, there is an immediate increase in the radius of the afferent arteriole, leading to a brief increase in blood flow. However, this stretch of the smooth muscle in the arteriole triggers its contraction, reducing its diameter and increasing resistance, which returns blood flow to the control value within about 30 seconds.
What equation describes the relationship between blood flow, pressure, and resistance?
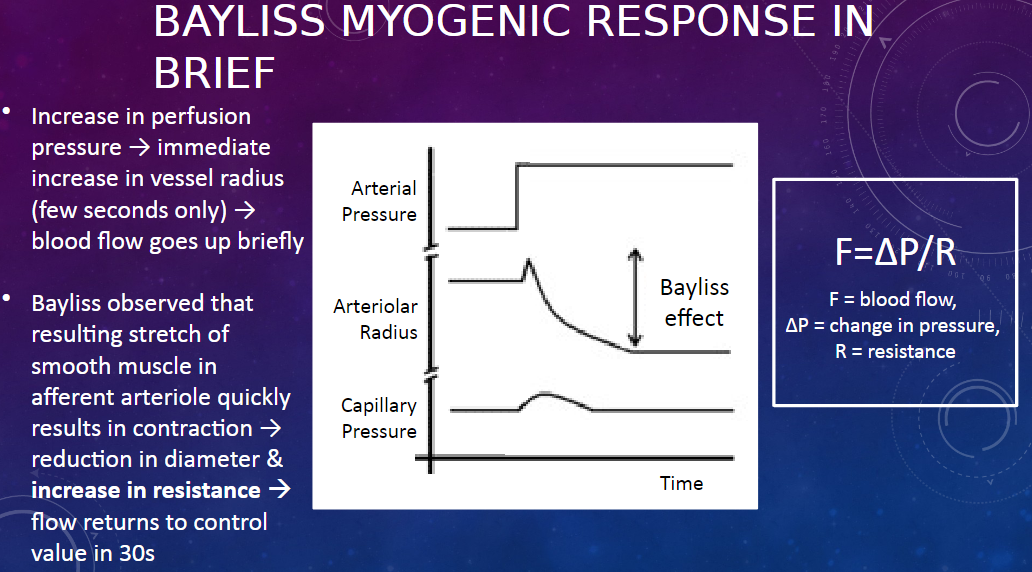
𖹭 The equation is F = ∆P / R, where F represents blood flow, ∆P represents the change in pressure, and R represents resistance.
What happens to the afferent arteriole during the Bayliss myogenic response?
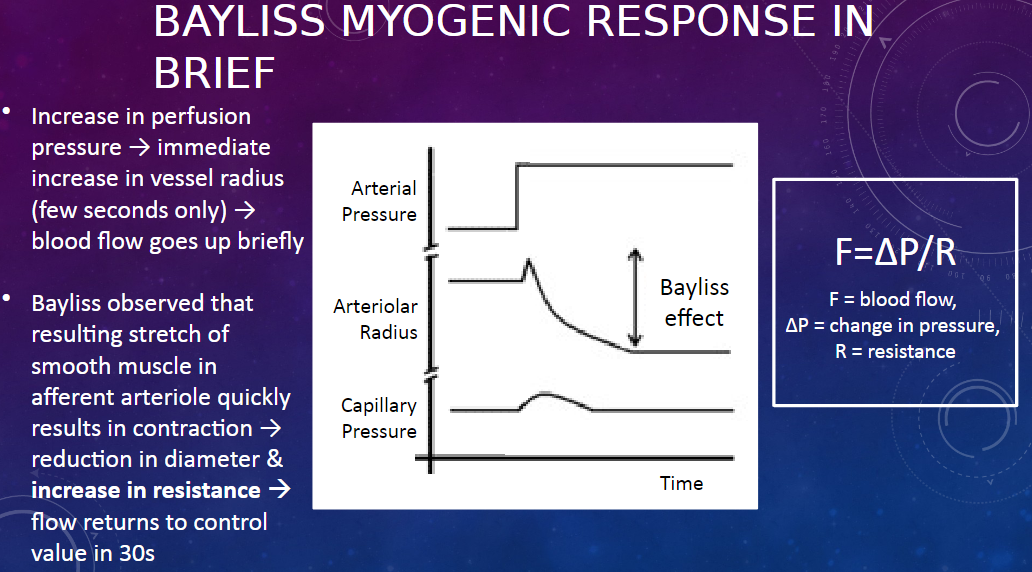
𖹭 During the Bayliss myogenic response, the afferent arteriole initially dilates in response to increased perfusion pressure, leading to increased blood flow. However, this dilation triggers smooth muscle contraction in the arteriole, reducing its diameter and increasing resistance, which restores blood flow to its control value.
Picture demonstrating ‘MYOGENIC RESPONSE’ OF AFFERENT ARTERIOLES TO PRESSURE IS ONE CAUSE OF AUTOREGULATION
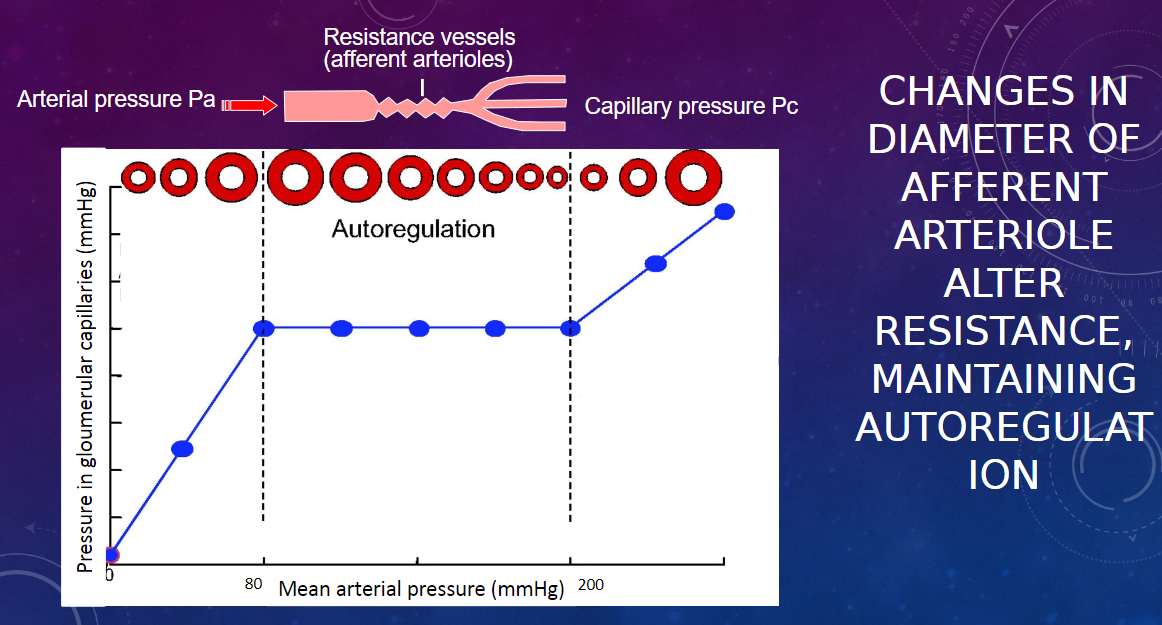
Picture demonstrating HOW CONTRACTION OF AFFERENT ARTERIOLEREGULATES GLOMERULAR CAPILLARY PRESSURE(FILTRATION FORCE):
What are the players involved in the tubuloglomerular feedback mechanism?
𖹭 The players are the macula densa and juxtaglomerular (JG) cells.
Describe the macula densa.
𖹭 The macula densa consists of specialized epithelial cells located in the distal convoluted tubule near the glomerulus.
What are JG cells?
𖹭 JG cells are modified smooth muscle cells found in the walls of the afferent arteriole proximal to the glomerulus.
What is the role of JG cells in the tubuloglomerular feedback mechanism?
𖹭 JG cells store inactive pro-renin and play a key role in regulating renal blood flow and glomerular filtration rate.
How do macula densa cells detect increased flow?
𖹭 Macula densa cells detect increased flow mechanically via cell surface cilia and chemically via increased sodium chloride concentration ([NaCl]).
What are the mechanisms through which macula densa cells detect increased flow?
𖹭 The mechanisms include mechanical detection via cell surface cilia and chemical detection via elevated sodium chloride concentration ([NaCl]).
What triggers the contraction of the afferent arteriole by macula densa cells?
𖹭 Macula densa cells release ATP, which triggers the contraction of the afferent arteriole.
What mechanism regulates glomerular filtration rate (GFR) and renal blood flow (RBF) through the tubuloglomerular feedback (TGF) system?
![<p>𖹭 Change in flow and sodium chloride concentration ([NaCl]) in the distal convoluted tubule (DCT) are sensed by the macula densa, which induces the release of paracrine vasoactive agents, leading to changes in the diameter and resistance of the afferent arterioles. This ultimately restores RBF and GFR.</p>](/flashcards/cardimage2/fc1ae2d5/353/8353956_back.png)
𖹭 Change in flow and sodium chloride concentration ([NaCl]) in the distal convoluted tubule (DCT) are sensed by the macula densa, which induces the release of paracrine vasoactive agents, leading to changes in the diameter and resistance of the afferent arterioles. This ultimately restores RBF and GFR.
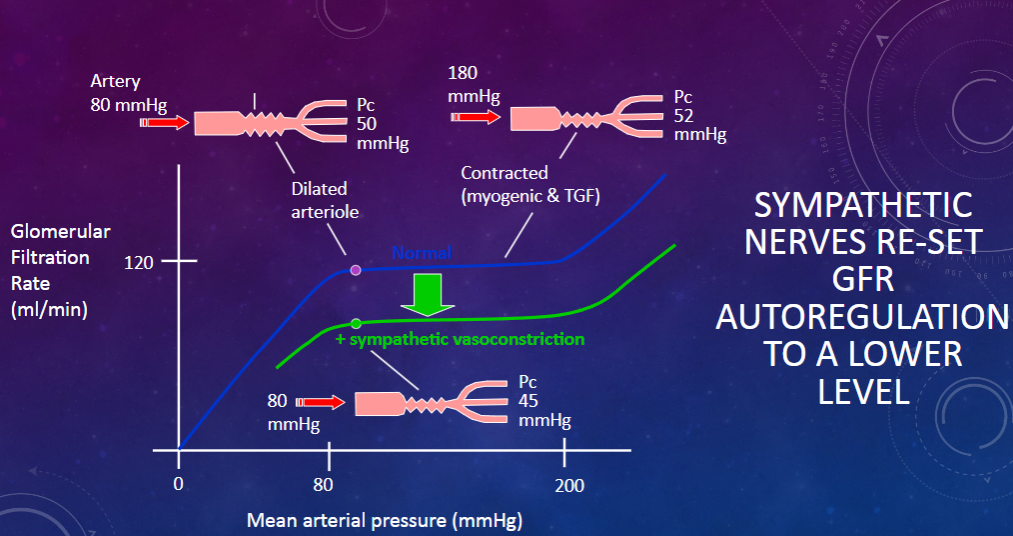
What are the conditions and mechanisms involved in the extrinsic control of glomerular filtration rate (GFR)? (3)
𖹭 Renal sympathetic nerves, which release noradrenaline and induce vasoconstriction, can reduce GFR by re-setting autoregulation to a lower level.
𖹭 This occurs in conditions such as standing upright (orthostasis), heavy exercise, and haemorrhage or other forms of clinical shock.
𖹭In shock, these sympathetic actions are further aided by circulating vasoconstrictor hormones such as adrenaline, angiotensin, and vasopressin, all aimed at conserving body fluid volume during physical stress.
Picture demonstrating how SYMPATHETIC NERVES RE-SET GFR AUTOREGULATION TO A LOWER LEVEL:
What are the two major clinical disorders of the glomerular filtration rate (GFR)?
𖹭 The two major clinical disorders of the GFR are:
Increased permeability of glomeruli to plasma proteins:
Condition: Nephrotic syndrome (e.g., filtration slit disordered by nephrin deficiency)
Symptoms:
Proteinuria (excess protein in the urine)
Hypoproteinaemia (low levels of protein in the blood)
Oedema (swelling due to fluid retention)
Decreased GFR:
Condition: Chronic glomerulonephritis leading to nonfunctioning glomeruli
Criteria: When GFR is less than 30 ml/min, this condition is termed chronic renal failure.
Treatment: Respond well to steroids