Pure substance

A substance made of only one kind of matter and having definite properties
Element

A pure substance that cannot be broken down into other substances
Atom
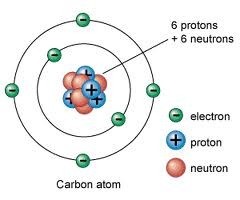
The basic particle from which all elements are made
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds
Heterogeneous mixture
A mixture in which you can see the different parts
Homogeneous mixture

A mixture in which you cannot see the different parts
Solution
A mixture that forms when one substance dissolves another.
Suspension
A mixture in which particles can be seen and easily separated by settling or filtration
Colloid
A mixture containing small, undissolved particles that do not settle out.
Physical property
A characteristic of a pure substance that can be observed without changing it into another substance
Viscosity

A liquid's resistance to flowing
Melting point
The temperature at which a solid becomes a liquid
Boiling point

The temperature at which a liquid changes to a gas
Filtration
A process that separates materials based on the size of their particles.
Distillation

The process of purifying a liquid by boiling it and condensing its vapors
Physical change
A change of matter from one form to another without a change in chemical properties
Chemical property
A characteristic of a pure substance that describes its ability to change into different substances
Chemical change
A change in matter that produces one or more new substances
Flammability

The ability of a substance to burn
Reactivity
How readily a substance combines chemically with other substances.
Precipitate
A solid that forms from a solution during a chemical reaction.