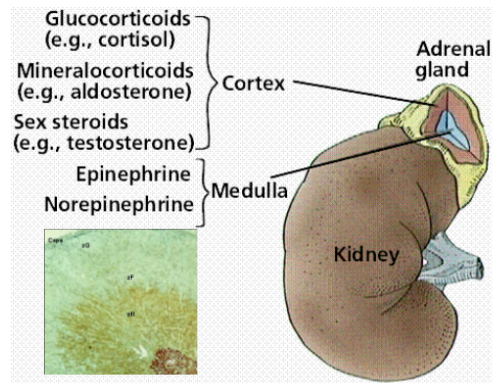
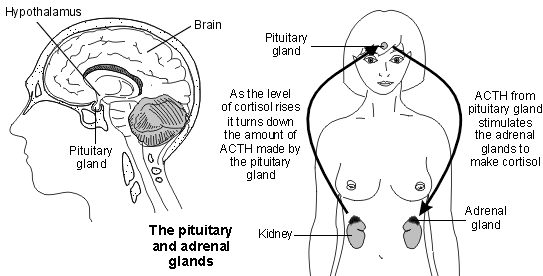
Picture demonstrating the hormones of the adrenal cortex:
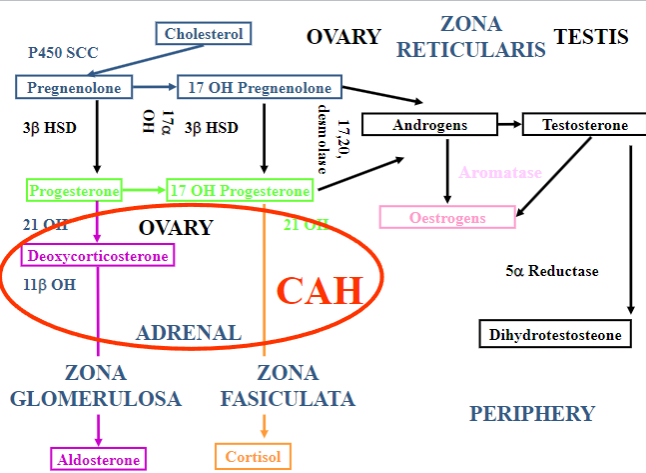
Picture demonstrating the mechanism of the hormones of the adrenal cortex:
What are the primary actions of cortisol on plasma glucose levels?
Cortisol increases plasma glucose levels by increasing gluconeogenesis, decreasing glucose utilization, increasing glycogenesis, and increasing glycogen storage.
How does cortisol affect lipid metabolism?
Cortisol increases lipolysis, providing energy.
What is the effect of cortisol on proteins?
Cortisol causes proteins to be catabolized, releasing amino acids.
How does cortisol impact sodium and water retention?
Cortisol promotes sodium and water retention, helping to maintain blood pressure.
What are the anti-inflammatory effects of cortisol?
Cortisol has anti-inflammatory properties.
What effect does cortisol have on gastric acid production?
Cortisol increases gastric acid production.
What is Cushing's Syndrome?
Cushing's Syndrome is a condition characterized by clinical features of chronic exposure to excessive levels of the steroid hormone cortisol.
Who first described Cushing's Syndrome and when?
Cushing's Syndrome was first described by Harvey Cushing in 1932.
What significant association did Harvey Cushing describe?
Harvey Cushing first described the association between pituitary gland tumours and signs of excess steroid hormones.
What is the incidence rate of Cushing's Syndrome?
The incidence rate of Cushing's Syndrome is 2 per 1,000,000 population.
At what age does Cushing's Syndrome typically onset?
Cushing's Syndrome typically onsets between 20-40 years old.
What is Cushing's Syndrome?
Cushing's Syndrome is characterized by excess cortisol in the blood.
What is Cushing's Disease?
Cushing's Disease is a specific form of Cushing's Syndrome caused by an ACTH-secreting pituitary tumor.
What are some clinical features of Cushing's Syndrome?
High blood pressure, fluid retention, depression, and anxiety.
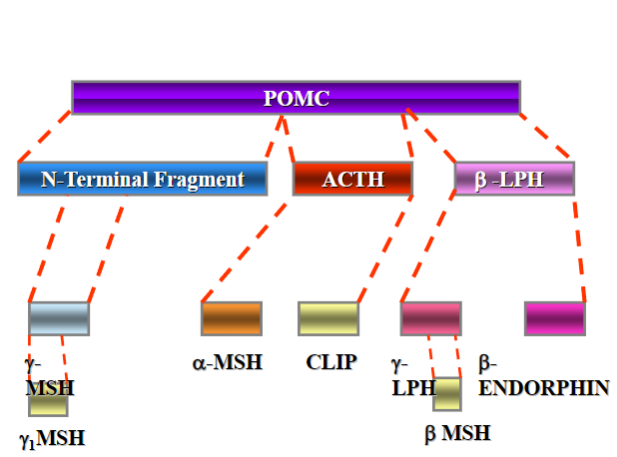
Picture demonstrating POMC (pro-opiomelanocortin) gene fragmentation:
What are the three stages in the investigation of Cushing’s Syndrome?
Screening, confirmation of the diagnosis, and differentiation of the cause.
What tests are involved in the screening stage for Cushing’s Syndrome?

Urinary free cortisol, diurnal rhythm, and overnight dexamethasone suppression test.
How is the diagnosis of Cushing’s Syndrome confirmed?

Low dose dexamethasone suppression testing.
How is the cause of Cushing’s Syndrome differentiated?
High dose dexamethasone suppression testing, ACTH, CRH test, and localisation.
What are the differential diagnoses for Cushing’s Syndrome?
Cushing’s Disease (pituitary adenoma), adrenal tumour (benign or malignant), and ectopic ACTH production (benign or malignant).
What are the laboratory features of Cushing’s Syndrome?
Hypokalaemia, metabolic alkalosis, and hyperglycaemia.
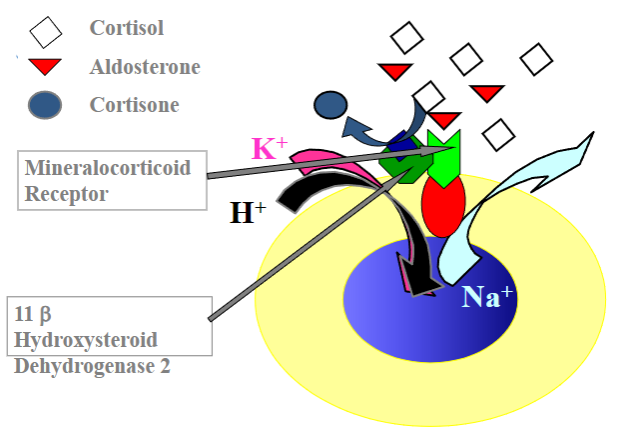
Picture demonstrating the action of the 11 betaHydroxysteroid Dehydrogenase 2:
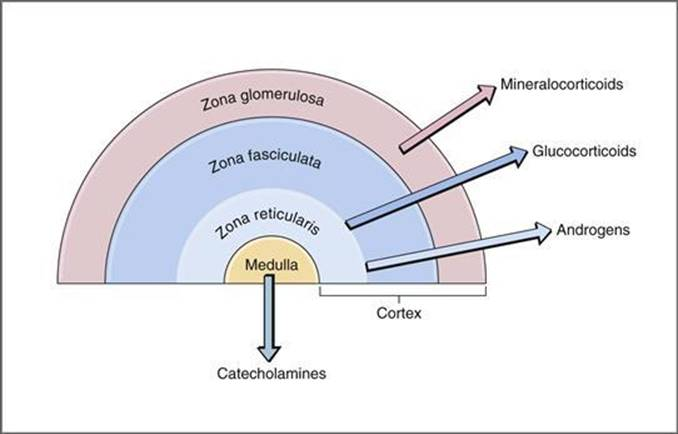
What hormones are produced by the adrenal cortex?
Glucocorticoids (cortisol), mineralocorticoids (aldosterone), and androgens.
What are the clinical features of Hashimoto’s primary hypothyroidism?
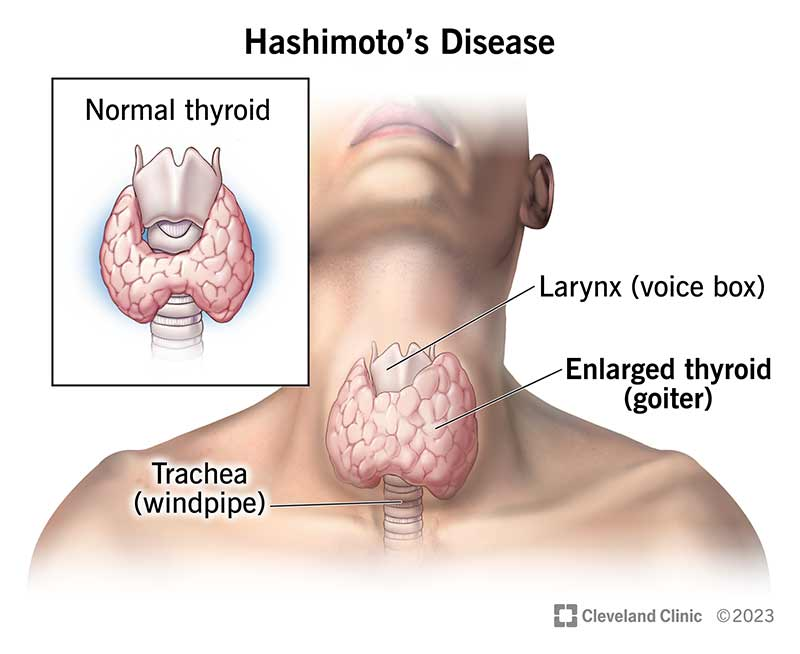
Autoimmune condition, low circulating thyroid hormone (TH), high TSH, lethargy, intolerance to cold, lack of growth and development, and diffuse goitre.
What is the blood flow pattern in the adrenal glands?
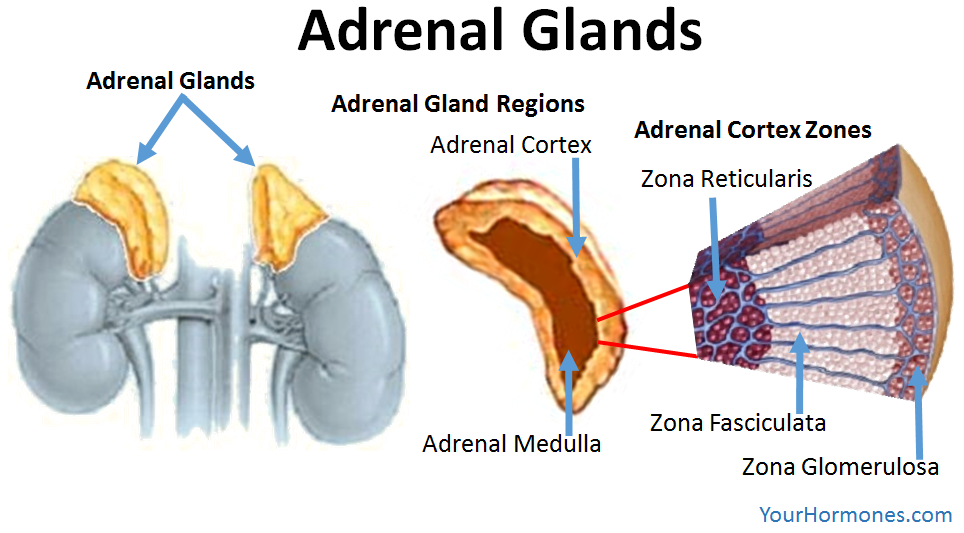
Blood flows from the outer cortex to the inner medulla, with layer-specific enzymes and functional zonation affecting hormone synthesis.
What are the primary actions of adrenal steroids?
Mineralocorticoids regulate salt and water balance, glucocorticoids affect metabolism and immune function, and androgens are weak androgens.
How is cortisol prevented from causing salt and water retention?
Cortisol is rapidly metabolized to inactive cortisone in the kidney by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2).
What are the main functions of glucocorticoids?
Decrease glucose utilization, promote lipolysis, stimulate gluconeogenesis, increase proteolysis, maintain blood glucose levels, ensure vascular integrity, and have anti-inflammatory and immunosuppressive effects.
What is ACTH and how is it related to the melanocortin receptors?
ACTH is synthesized from pro-opiomelanocortin (POMC) and binds to melanocortin receptors. Excess ACTH can cause skin pigmentation.
What are the clinical features of Addison’s disease (primary adrenal insufficiency)?
Low circulating adrenal steroids, high ACTH, normal to low plasma sodium, normal to high plasma potassium, and elevated plasma renin.
What are the types of adrenal insufficiency and their causes?
Addison’s disease (primary), hypopituitarism (secondary hypocortisolism), failure in RAAS (secondary hypoaldosteronism), and enzyme defects in steroid synthesis pathways.
What is Cushing’s syndrome and its types?
Hypercortisolism due to excess glucocorticoid. It can be ACTH-dependent (Cushing's disease, ectopic ACTH-secreting tumor) or ACTH-independent (adrenal adenoma or carcinoma, iatrogenic).
What are the clinical features of hypercortisolism (Cushing’s syndrome)?
Hypertension, hyperglycaemia, truncal obesity, fatigue, muscle weakness, virilization, depression, and mood disturbances.
What is the first step in diagnosing Cushing’s syndrome?
Confirm hypersecretion of cortisol using 24-hour urinary cortisol and cortisol at nadir of secretion.
What is the dexamethasone suppression test used for in Cushing’s syndrome diagnosis?
To determine the cause of hypercortisolism by assessing ACTH levels and response to dexamethasone suppression.
What is the primary treatment for an adrenal adenoma in Cushing's syndrome?
Surgery and cortisol production blockers such as Metyrapone and Ketoconazole.
What are the treatment options for adrenal cancer in Cushing's syndrome?
DXT/Deep X-Ray Therapy (three field or gamma knife) and CXT/Chest Radiography (Mitotane).
What treatments are available for pituitary-related Cushing's syndrome?
Transsphenoidal surgery (TSS) and DXT. Patients may also require replacement of other pituitary hormones following treatment.
What medication is necessary for patients at the time of and following surgery for Cushing's syndrome?
Steroid replacement tablets.
Why do patients need steroid replacement tablets after surgery for Cushing's syndrome?
The adrenal tumour suppresses the function of the normal gland.
Will patients need steroid replacement tablets long-term after treatment for Cushing's syndrome?
Many patients will not need the steroid tablets long-term.
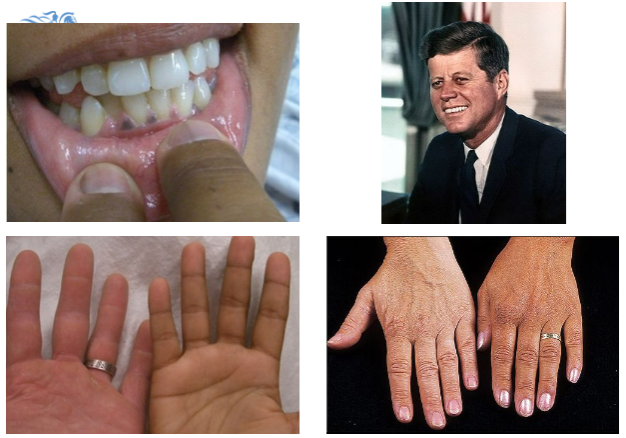
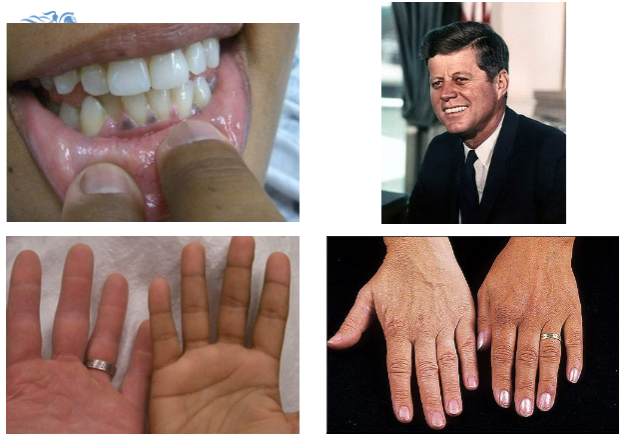
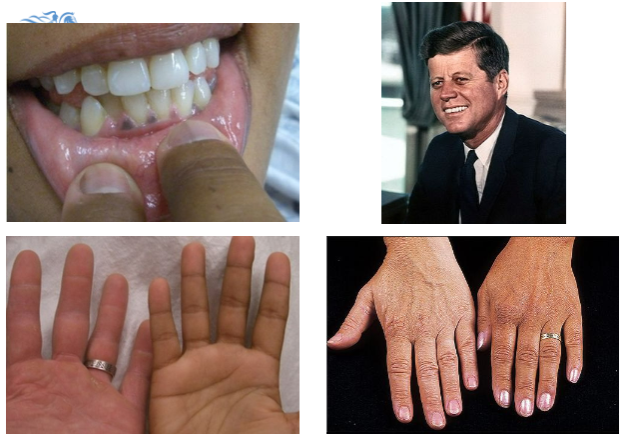
What are the common symptoms of Addison's disease?
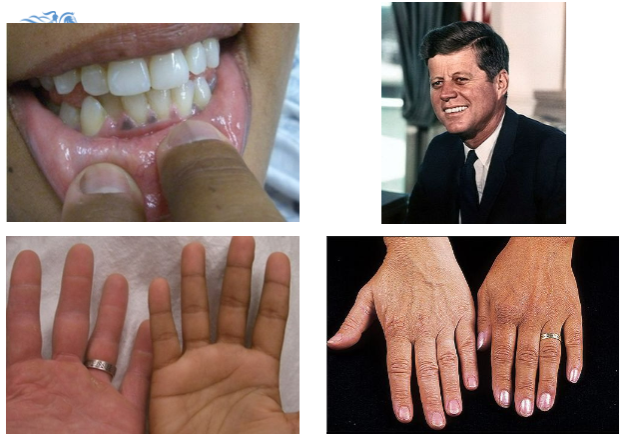
Tiredness, weakness, anorexia, weight loss, postural hypotension, myalgia, salt craving, nausea, vomiting, hyperpigmentation, and vitiligo.
What electrolyte imbalances are associated with Addison's disease?
Hyponatremia, hyperkalemia, and hypercalcemia.
What metabolic disturbances can occur in Addison's disease?
Acidosis and hypoglycemia.
What laboratory findings are commonly seen in Addison's disease?
Increased urea and creatinine, eosinophilia, and lymphocytosis.
What are the autoimmune-related causes of Addison's Syndrome?
Autoimmune disorders.
What infectious causes can lead to Addison's Syndrome?

TB (tuberculosis), fungal histoplasmosis, viral HIV, and bacterial TB.
What are the potential causes of Addison's Syndrome related to infiltration?
Amyloidosis, haemochromatosis, Waterhouse-Freidrichson syndrome, and apoplexy.
What congenital conditions can result in Addison's Syndrome?
Congenital adrenal hyperplasia, adrenoleukodystrophy, and adrenomyeloneuropathy.
What can cause Addison's Syndrome related to steroid withdrawal?
Withdrawal from long-term steroid therapy.
What are some other causes of Addison's Syndrome?
Metastases, infection, and drugs.
What tests are commonly used to investigate adrenal insufficiency?
9 AM cortisol, ACTH (adrenocorticotropic hormone), electrolytes, FBC (full blood count), adrenal imaging, adrenal antibodies, investigations for other causes of adrenal failure, infection screen, imaging for cancer, and biochemical testing for enzyme deficiency.
What is the significance of measuring 9 AM cortisol in testing for adrenal insufficiency?
It helps assess cortisol levels, which are typically highest in the morning.
Why is ACTH testing important in the investigation of adrenal insufficiency?
ACTH levels can indicate whether adrenal insufficiency is primary (due to adrenal gland dysfunction) or secondary (due to pituitary or hypothalamic dysfunction).
What imaging studies are recommended for investigating adrenal insufficiency?
Adrenal imaging and imaging for cancer to assess for any structural abnormalities or malignancies.
Why are electrolyte levels checked in testing for adrenal insufficiency?
Adrenal insufficiency can lead to electrolyte imbalances, particularly hyponatremia and hyperkalemia.
What additional tests might be conducted to investigate the underlying cause of adrenal insufficiency?
Adrenal antibodies, infection screen, and biochemical testing for enzyme deficiency to identify specific causes such as autoimmune disorders or enzyme deficiencies.
What dynamic tests are used to assess adrenal function?
Short Synacthen test (250mcg), Long Synacthen test (1mg), insulin tolerance test, and glucagon test.
What is the purpose of the Short Synacthen test in adrenal insufficiency diagnosis?
It evaluates adrenal response to ACTH stimulation by administering synthetic ACTH (Synacthen) and measuring cortisol levels.
How does the Long Synacthen test differ from the Short Synacthen test?
The Long Synacthen test uses a higher dose of Synacthen (1mg) and measures cortisol levels over a longer period, providing more comprehensive assessment of adrenal function.
What is the treatment protocol for adrenal insufficiency?
Hydrocortisone (10mg in the morning, 5mg at noon, and 5mg in the afternoon) to mimic the diurnal rhythm, with the last dose before 6pm. Fludrocortisone (50-200mcg once daily) may also be prescribed.
What is congenital adrenal hyperplasia (CAH)?
CAH is a group of autosomal recessive disorders characterized by enzyme deficiencies in cortisol biosynthesis, leading to adrenal gland hyperplasia and impaired cortisol and aldosterone production.
What is the most common form of CAH?
21-hydroxylase deficiency is the most common form of CAH.
It is an inherited disorder caused by mutations in the CYP21A2 gene, which encodes the enzyme 21-hydroxylase. This enzyme is essential for cortisol synthesis, and its deficiency leads to impaired cortisol production.
What is the incidence of classical CAH?
Approximately 1 in 10,000 live births.
What genetic factors are associated with classical CAH?

It is autosomal recessive and has an increased incidence in populations with specific HLA haplotypes, such as HLA-A3, Bw47, and DR7, with a higher prevalence observed in certain ethnic groups like Yupik Eskimos.
Picture demonstrating what happens when 21-hydroxylase is deficient:
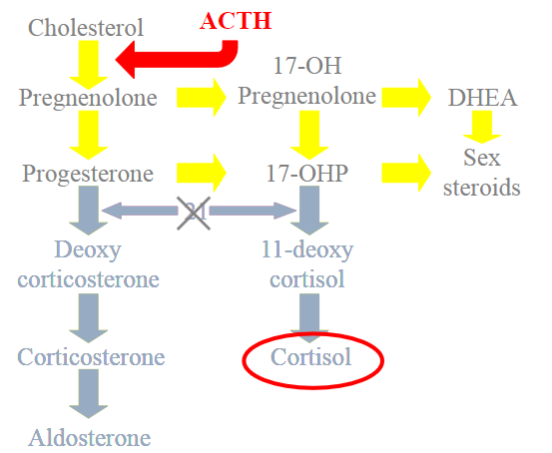
What are the clinical features of 21-hydroxylase Deficiency (Classical)?

Excess sex steroids can cause virilization, hirsutism, premature adrenarche, and infertility. Additionally, due to the lack of aldosterone, patients may experience salt-losing crisis characterized by hyperkalemia and hypotension.
What are the key features of 11β-hydroxylase Deficiency (Non-Classical) CAH?

It accounts for approximately 5% of reported cases of congenital adrenal hyperplasia (CAH).
The incidence is around 0.5 per 100,000 live births, and it follows an autosomal recessive inheritance pattern.
There's an increased prevalence among Moroccan Jews, with an incidence of 1 in 6,000 live births.
It's also linked to specific HLA haplotypes, including HLA-B14 and DR1.
What are the clinical features associated with 11β-hydroxylase Deficiency (Non-Classical)?
Excess sex steroids can result in virilization, hirsutism, premature adrenarche, and infertility. Additionally, due to the lack of aldosterone but high levels of deoxycorticosterone (DOC), patients may experience hypertension and hypokalemia.
What investigations are typically performed to diagnose 11β-hydroxylase Deficiency?
Synacthen (ACTH Stimulation) Test:
What it reveals: When given synthetic ACTH (Synacthen), patients with 11β-hydroxylase deficiency do not show an increase in cortisol levels. Instead, they have high levels of 17-hydroxyprogesterone (17-OHP), a precursor hormone.
Prednisolone Suppression Test:
What it reveals: Administering prednisolone, a type of glucocorticoid, should lower androgen levels to within the normal range. This is because prednisolone reduces ACTH production, which in turn decreases the production of excess androgens.
What is the mainstay of treatment for 11β-hydroxylase Deficiency?
Glucocorticoid therapy is primarily used to replace cortisol and inhibit ACTH production, thus reducing adrenal testosterone production.
What additional treatments might be necessary for individuals with 11β-hydroxylase Deficiency?
Surgery may be required for virilized female genitalia. Treatment of the mother may be necessary to prevent fetal virilization. Fludrocortisone is utilized to replace absent mineralocorticoid activity.
Where is aldosterone primarily produced?
Aldosterone is primarily produced in the zona glomerulosa of the adrenal cortex.
How does aldosterone act on the kidney?
Aldosterone acts on the kidney via receptors and binds glucocorticoids with equal affinity.
What type of receptor does aldosterone primarily interact with?
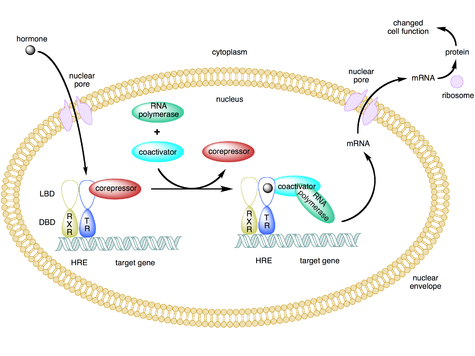
Aldosterone primarily interacts with intranuclear receptor type 1.
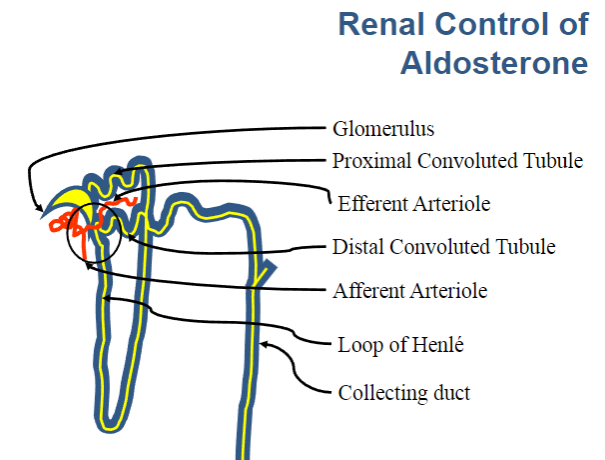
Picture demonstrating the nephron:
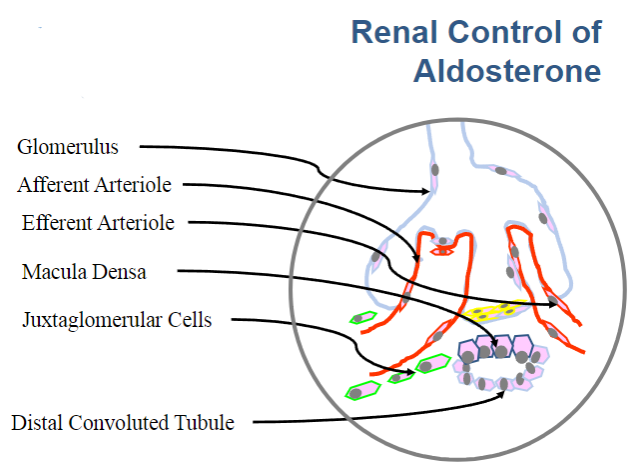
Picture demonstrating the glomerulus:
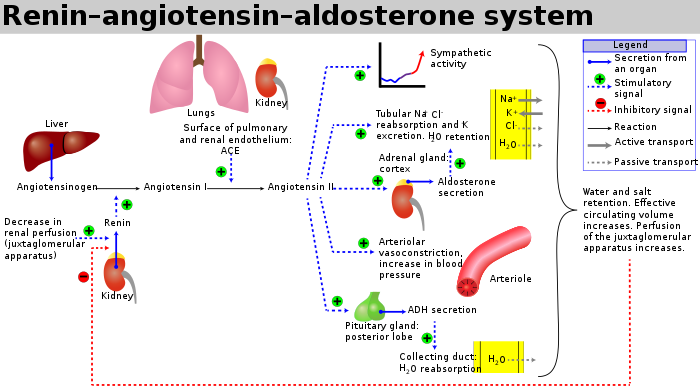
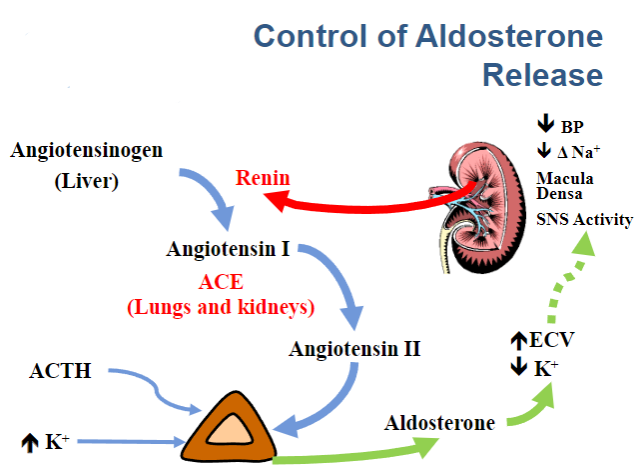
Picture demonstrating the control of aldosterone release:
What are the characteristics of primary aldosterone excess?
Primary aldosterone excess is characterized by high aldosterone levels and low renin levels.
What are some conditions associated with primary aldosterone excess?
Conditions include Conn's Syndrome, bilateral adrenal hyperplasia, steroid-treatable hypertension, and aldosterone-producing adrenal carcinoma.
What distinguishes secondary aldosterone excess from primary?
Secondary aldosterone excess is characterized by high aldosterone levels and high renin levels.
What are some conditions associated with secondary aldosterone excess and hypertension?
Conditions include renal artery stenosis, renin-secreting tumours, and malignant nephrosclerosis.
In which conditions is aldosterone excess present but blood pressure remains normal?
Aldosterone excess can be present without hypertension in conditions such as congestive heart failure (CCF), cirrhosis, nephrotic syndrome, and dehydration.
What are the treatment options for Conn's syndrome?
Treatment options include Spironolactone or Eplerenone, Amiloride or Triampterine, potassium supplementation, and surgery to address the primary tumour.
What are the key points summarized about adrenal disorders?
Adrenal glands comprise two parts: the adrenal cortex and the adrenal medulla. Adrenal overactivity can manifest as Cushing’s syndrome, Conn’s syndrome, or adrenal hyperplasia. Adrenal underactivity includes Addison’s disease and congenital adrenal hyperplasia (CAH).
What is a phaeochromocytoma?
Phaeochromocytoma is a tumor of the enterochromaffin cells of the adrenal medulla.
Where can extra-adrenal phaeochromocytoma occur?
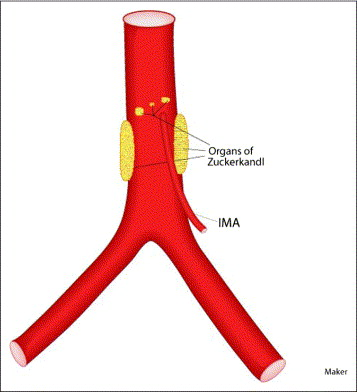
Extra-adrenal phaeochromocytoma can occur in sympathetic ganglia or the organ of Zuckerkandl.
What are the catecholamines produced by phaeochromocytoma?
Phaeochromocytoma can produce epinephrine, norepinephrine, and dopamine.
What is the "Rule of 10" associated with phaeochromocytoma?
The "Rule of 10" states that approximately 10% of phaeochromocytomas are bilateral, 10% are malignant, and 10% are extra-adrenal.
What is known about the genetics of phaeochromocytoma?

Current estimates suggest that over 40% of phaeochromocytomas are related to an inherited germline mutation, while more than 30% are associated with a somatic mutation.
Picture demonstrating Pheochromacytoma Syndromes:
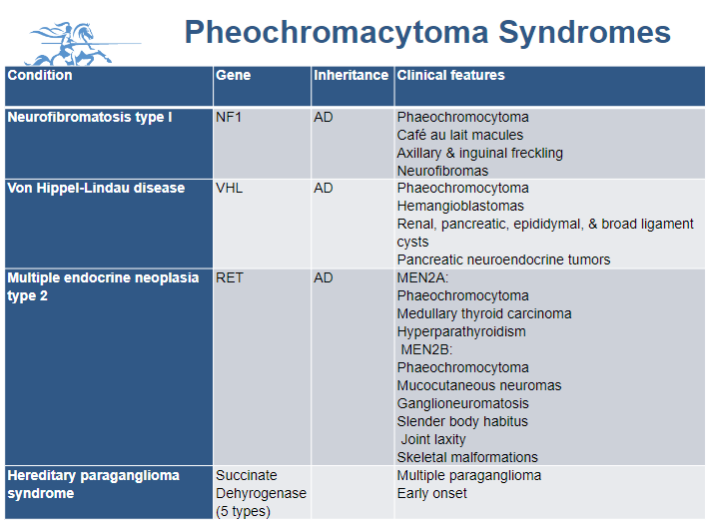
What are the common symptoms of Phaeochromocytoma?
Symptoms include sweating, headache, tachycardia, anxiety, fever, paroxysmal hypertension, constipation, abdominal pain, fits, visual disturbance, pallor or flushing, palpitation, and sometimes hypotension or angor animii.
What laboratory findings are associated with Phaeochromocytoma?
Laboratory findings may include eosinophilia, hyperglycemia, and hypercalcemia. Urinary tests may reveal elevated levels of metanephrines and catecholamines.
What imaging modalities are used in the investigation of Phaeochromocytoma?
Imaging modalities include CT, MRI, MIBG scan (I131 meta-iodobenzylguanidine), and 68 Ga-DOTA-NOC PET CT scan.
What is the emergency management approach for Phaeochromocytoma?
Emergency management includes alpha blockade with non-competitive alpha antagonists like phenoxybenzamine or phentolamine, followed by beta blockade with nonselective beta blockers such as propranolol. Fluid resuscitation is also essential.
What medications are used for alpha blockade in Phaeochromocytoma?
Alpha blockade can be achieved with non-competitive alpha antagonists like phenoxybenzamine or phentolamine, as well as competitive antagonists like doxazosin.
What medications are used for beta blockade in Phaeochromocytoma?
Beta blockade is typically achieved with nonselective beta blockers such as propranolol.
What is the definitive treatment for Phaeochromocytoma?
Surgery is the definitive treatment for Phaeochromocytoma.
What are the summarized points regarding adrenal disorders?
Adrenal glands consist of two parts: the adrenal cortex and the adrenal medulla. Adrenal overactivity can manifest as Cushing’s syndrome, Conn’s syndrome, adrenal hyperplasia, and Phaeochromocytoma. Adrenal underactivity includes Addison’s disease, congenital adrenal hyperplasia (CAH), and secondary adrenal failure.