An object has a mass of 100.0 grams. The object's volume is 20.0 cubic centimeters. What is the object's density
5.0 grams/cm^3
The object's dimensions are 10.0 cm x 2.0 cm x 2.0 cm. Calculate the object's volume.
40 cm^3
A rock has a mass of 77.0 grams. The volume is found using water displacement. The initial volume of water is 10.0 mL and the volume of the rock and water is 17.0 mL. Determine the rock's density
11.0 grams/cm^3
Find the mass of a piece of gold that has a volume of 3.0 cm^3. The density of gold is 6.0 g/cm^3.
18.0 g
how much matter is in a given space
density
object sinks water
Density > 1.0 g/cm^3
object floats in water
Density < 1.0 g/cm^3
method used to determine volume of irregularly shaped objects
water displacement
device used to determine mass
balance
device used to determine liquid volumes
graduated cylinder
device used to measure lengths
ruler
1.0 g/cm^3
Density of water
Units used for volume of a regular object
cm^3
Units used for mass of an object
g
Units used for volume of an irregular object
mL
What is the total volume of an object that had an initial volume of 20 mL and a final volume of 27 mL?
7 mL
What is the total volume of an object that had an initial volume of 30 mL and a final volume of 45 mL?
15 mL
Equation for volume of a regular object
l x w x h
Equation for the volume of an irregular object
final volume - initial volume
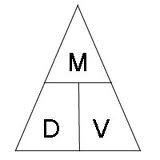
Density Equation
d= m/v
M = 48g, V = 8cm3 D = ?
6 g/cm3
D = 7g/cm3, V = 3 cm3 m = ?
21 g
Most dense in water will be...
at the bottom
Mass =
Volume/Density
Volume =
Mass/Density
A cube with a length of 2 cm has a mass of 16 g. What is the cubes density?
2 g/cm^3
A measure of the force of gravity on an object
Weight
the amount of matter in an object
Mass
The amount of space an object takes up
Volume
Density Triangle