What is motor control? (2)
Motor control refers to brain mechanisms that produce purposeful, coordinated movements.
The brain adapts motor outputs to respond to changing sensory inputs from the body and the external environment.
Which structures of the brain are involved in motor control? (7)
Motor Cortices: M1, SMA, preSMA, premotor cortex
Brainstem Centres
Spinal Cord
Parietal Cortex
Prefrontal Cortex
Cerebellum
Basal Ganglia
What are the Brain structures included in the Basal Ganglia (4)
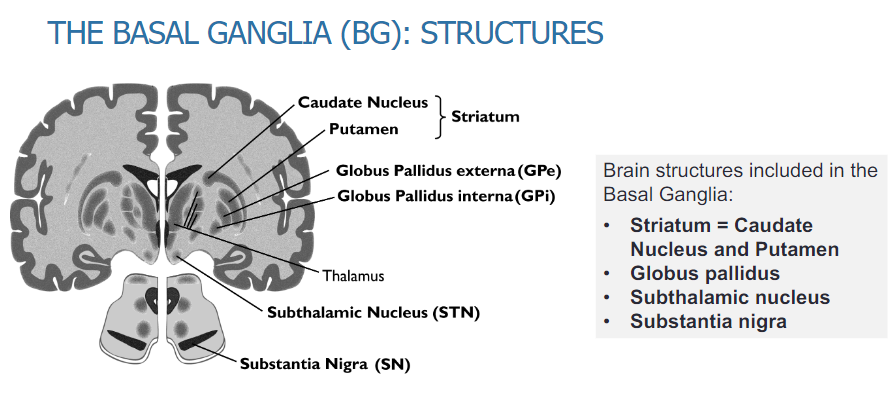
Striatum: Caudate Nucleus and Putamen
Globus Pallidus
Subthalamic Nucleus
Substantia Nigra
What are the major structures of the basal ganglia and their roles (4)?
Striatum (Caudate Nucleus, Putamen)
Globus Pallidus
Subthalamic Nucleus
Substantia Nigra
What are the major afferents to the basal ganglia (1)?
Primary input pathways to the basal ganglia (cerebral cortex, thalamus and brainstem).
What are the intrinsic connections within the basal ganglia (1)?
Connections between the various basal ganglia structures.
What are the efferent connections of the basal ganglia (1)?
Output pathways from the basal ganglia to the thalamus and motor cortex.
What is the role of the basal ganglia in motor control (3)?
Initiation of movement
Focusing function
Scaling movement (amplitude, velocity, rhythm)
Where does the basal ganglia receive inputs from and send outputs to (2)?
Inputs from multiple cortical and brainstem regions
Outputs to selected parts of these same areas
What are the main functions of the basal ganglia (3)?
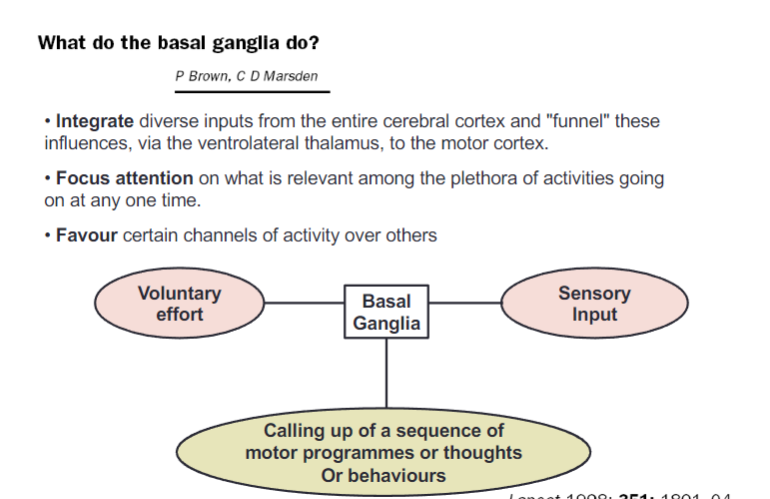
Integrate diverse inputs from the entire cerebral cortex and "funnel" these influences, via the ventrolateral thalamus, to the motor cortex
Focus attention on what is relevant among the plethora of activities occurring at any one time
Favor certain channels of activity over others
How do the basal ganglia contribute to motor and cognitive processes (3)?
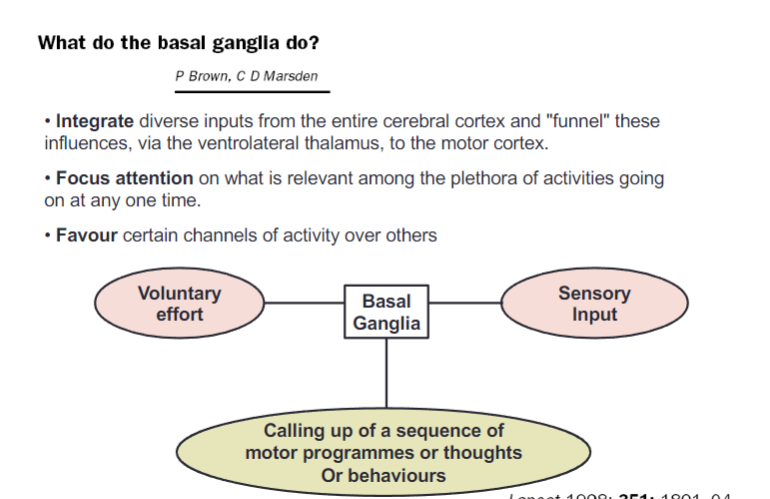
Voluntary effort is coordinated with basal ganglia processing
Sensory input is integrated to refine actions
Sequences of motor programs, thoughts, or behaviors are called up
What are movement disorders associated with abnormal function of the basal ganglia (10)?

Hypokinetic movement disorders (MDS):
Slowed or absent voluntary movements (hypokinesia)
Parkinsonism:
Parkinson's Disease
Atypical Parkinsonisms
Hyperkinetic movement disorders (MDS):
Excessive and involuntary movements (hyperkinesia)
Tremor
Dystonia
Chorea
Tics
Myoclonus
What are the core features of motor disorder in Parkinson's disease (5)?

Bradykinesia:
Progressive reduction of amplitude
Progressive reduction of speed
Lack of spontaneous movements (oligokinesia)
Difficulty executing internally generated movements
Difficulty with externally paced movements
What is the function of the basal ganglia? (2)
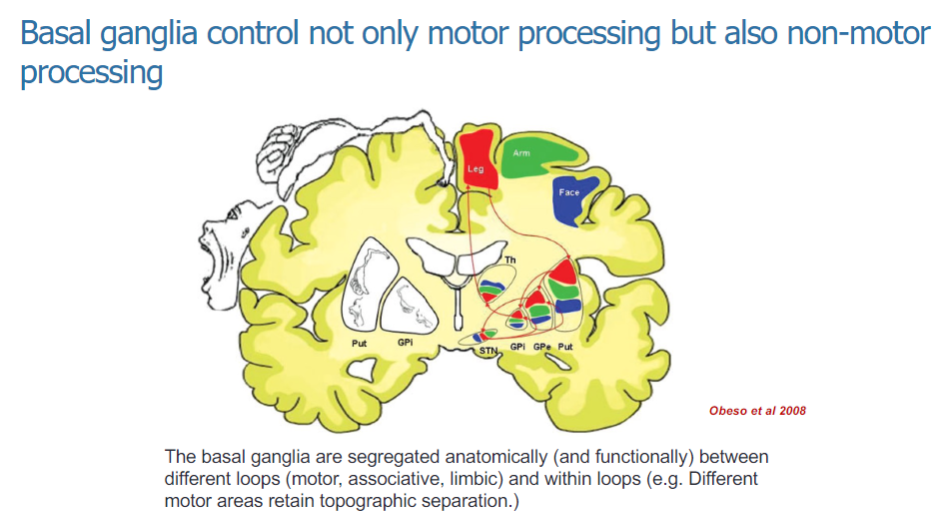
The basal ganglia control both motor and non-motor processing.
They are involved in the regulation of various functions, including movement and cognitive/emotional processing.
How are the basal ganglia organized anatomically and functionally? (3)
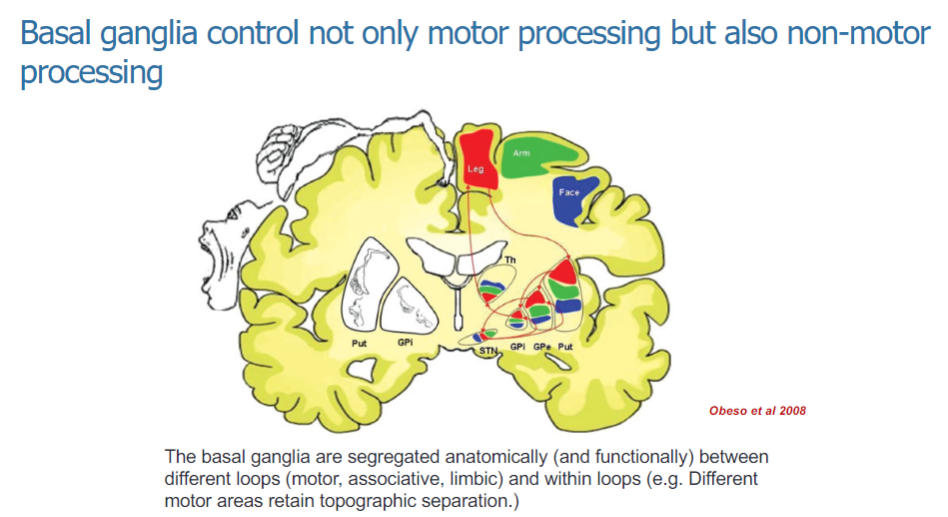
The basal ganglia are anatomically and functionally segregated into different loops: motor, associative, and limbic.
These loops help to process specific types of information related to movement and non-motor functions.
Within the motor loop, different motor areas maintain topographic separation, ensuring organized control of movement.
What are the non-motor functions sustained by the basal ganglia? (5)
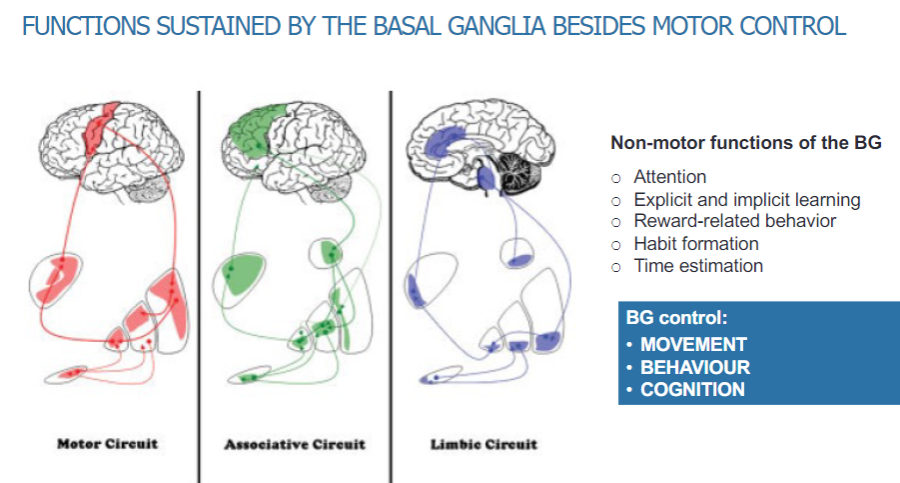
Attention
Explicit and implicit learning
Reward-related behaviour
Habit formation
Time estimation
What are the primary functions controlled by the basal ganglia? (3)

Movement
Behavior
Cognition
What are the different circuits within the basal ganglia? (3)
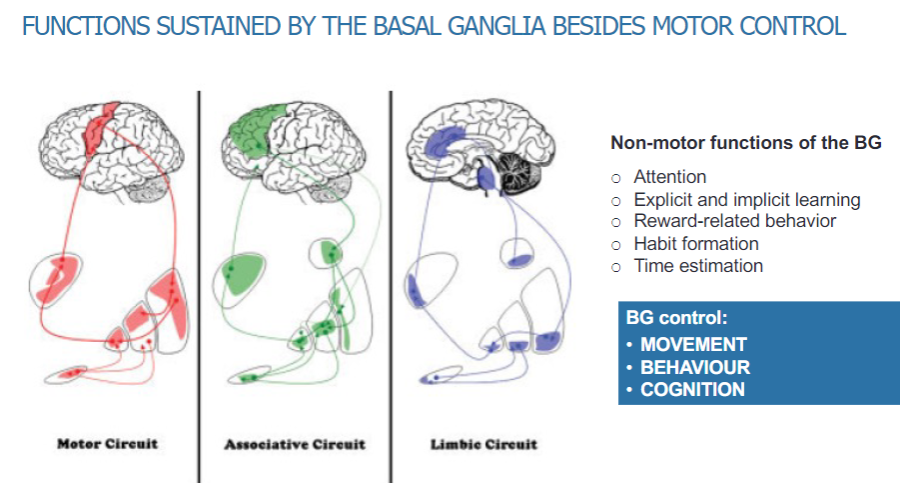
Motor circuit
Associative circuit
Limbic circuit
What is the main output of the basal ganglia and how does it affect movement? (2)
The main output of the basal ganglia is inhibitory.
Increased activity in the basal ganglia leads to less movement.
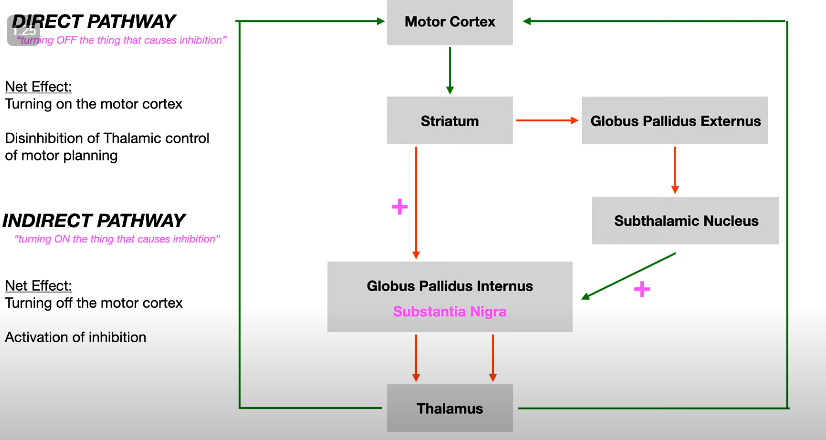
What are the two pathways through the basal ganglia and their effects on movement? (4)
One pathway decreases output activity, which increases movement.
The other pathway increases output activity, which decreases movement.
How does dopamine affect the pathways in the basal ganglia? (1)
Dopamine has different effects on the two pathways, influencing movement in distinct ways.
What is the Alexander and DeLong model of the basal ganglia? (4)
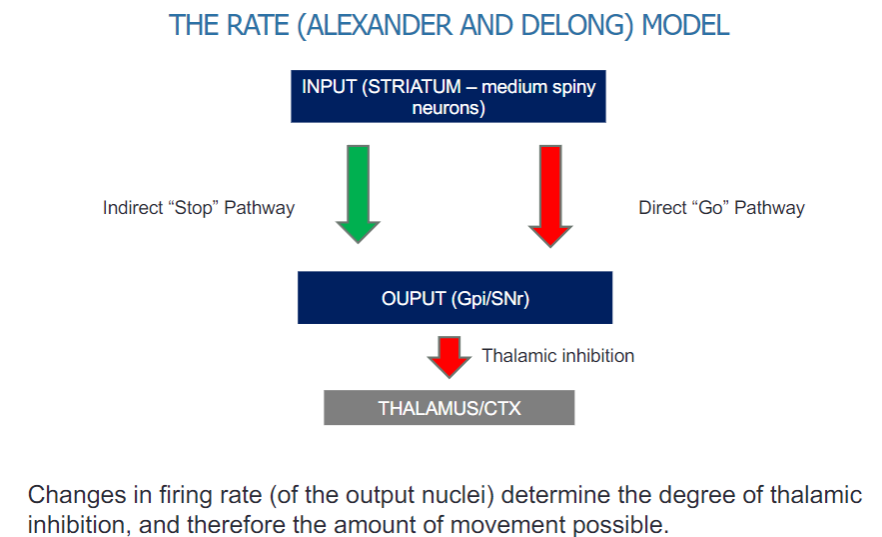
The model involves two main pathways:
Indirect "Stop" pathway
Direct "Go" pathway
The input comes from the striatum, specifically medium spiny neurons.
The output is directed through the globus pallidus internal segment (Gpi) and the substantia nigra pars reticulata (SNr).
How does the firing rate of output nuclei affect movement in the basal ganglia? (2)
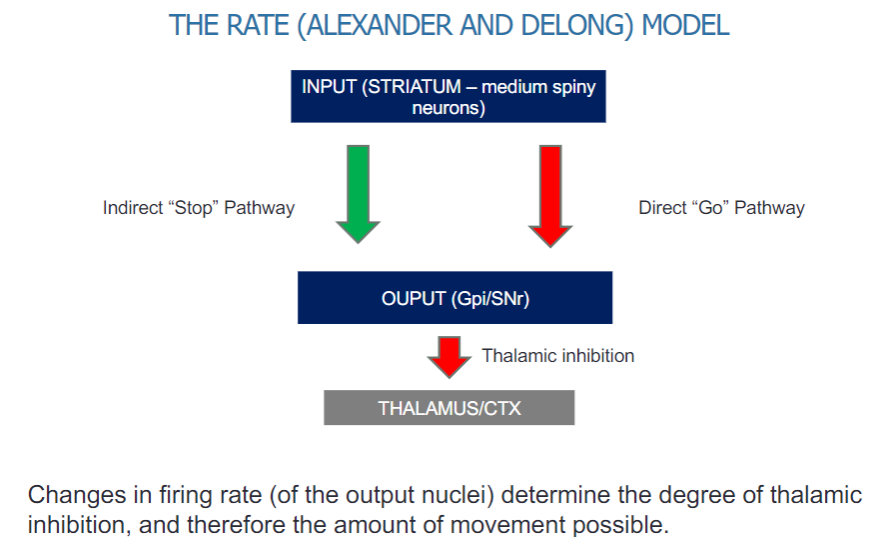
Changes in the firing rate of the output nuclei (Gpi/SNr) determine the level of thalamic inhibition.
This inhibition influences the amount of movement possible by regulating thalamic input to the cortex.
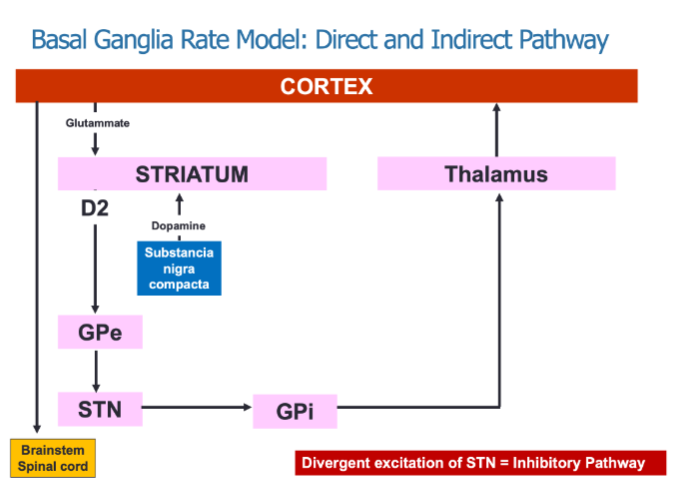
What is the role of the direct and indirect pathways in the Basal Ganglia Rate Model? (3)
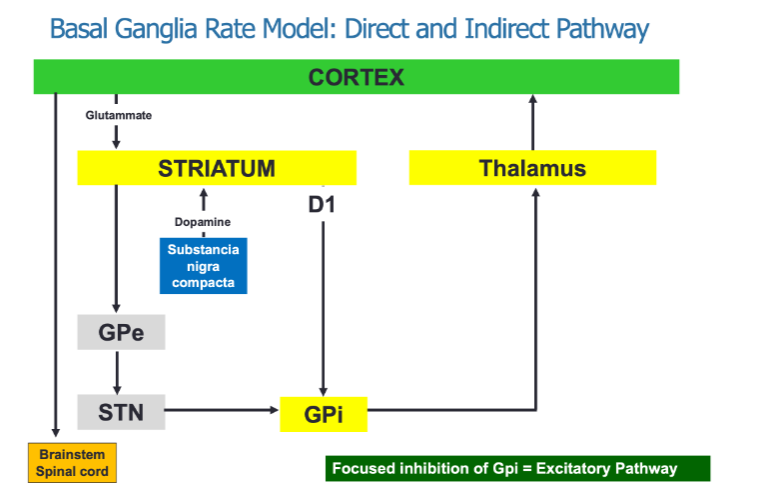
The direct pathway promotes movement through focused inhibition of the GPi (Globus Pallidus internal).
The indirect pathway inhibits movement by involving multiple structures, including the GPe (Globulus Pallidus external) and STN (Subthalamic nucleus).
Both pathways interact to regulate movement via thalamic output to the cortex.
How does dopamine influence the pathways in the basal ganglia? (2)
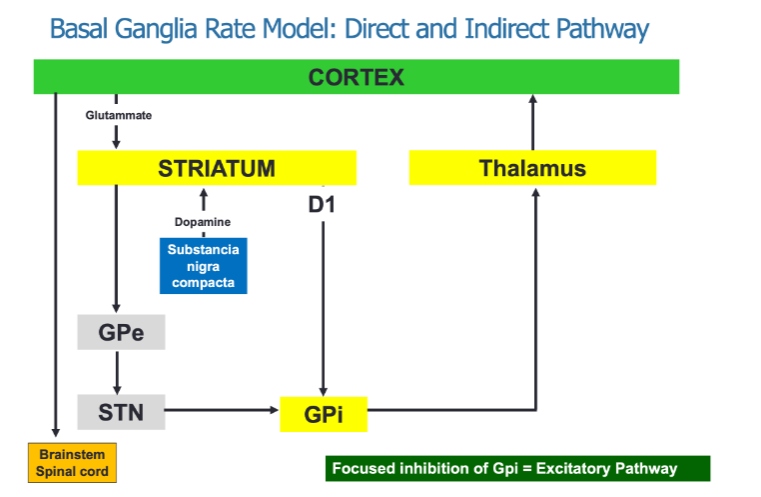
Dopamine acts on D1 receptors, which are part of the direct "Go" pathway, facilitating movement.
Dopamine also acts on D2 receptors, which are part of the indirect "Stop" pathway, inhibiting movement.
What neurotransmitters are involved in the basal ganglia's regulation of movement? (2)
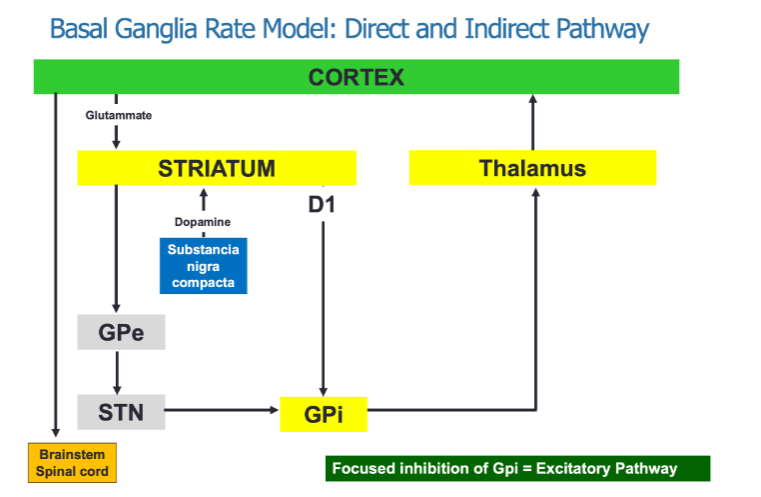
Glutamate from the cortex excites the striatum.
Dopamine from the substantia nigra compacta modulates the activity of both the direct and indirect pathways.
What is the role of the direct and indirect pathways in the Basal Ganglia Rate Model? (3)
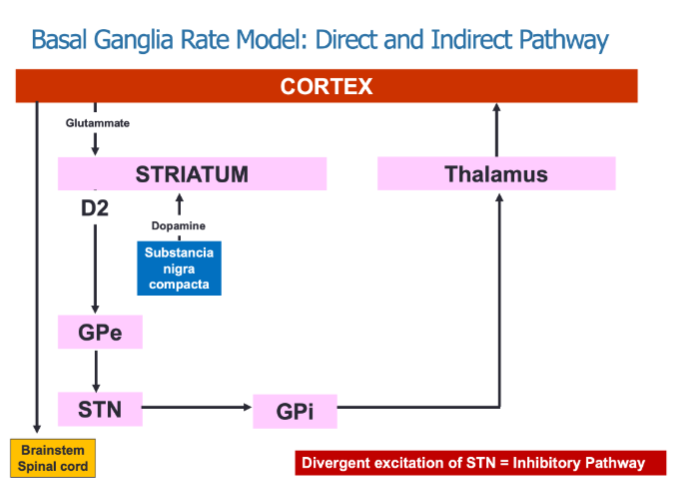
The direct pathway promotes movement by inhibiting the GPi, resulting in thalamic excitation.
The indirect pathway inhibits movement through divergent excitation of the STN, which increases inhibition of the GPi.
Both pathways work together to regulate motor activity by modulating thalamic input to the cortex.
How does dopamine influence the direct and indirect pathways? (2)
Dopamine acts on D1 receptors to facilitate the direct "Go" pathway, promoting movement.
Dopamine acts on D2 receptors to inhibit the indirect "Stop" pathway, allowing for movement.
What neurotransmitters are involved in the regulation of movement within the basal ganglia? (2)
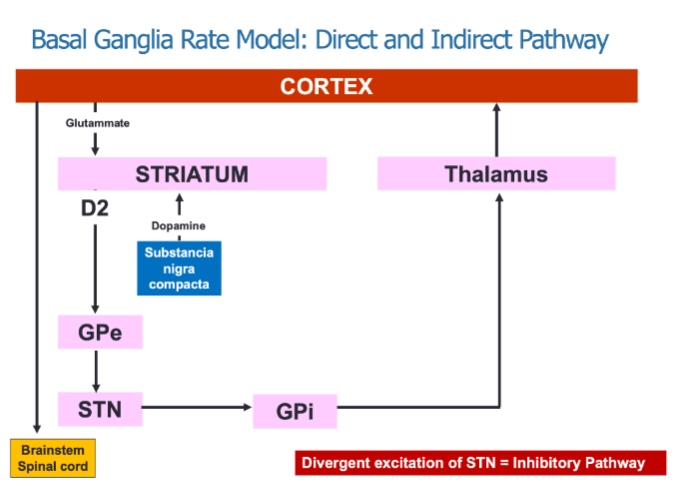
Glutamate from the cortex excites the striatum.
Dopamine from the substantia nigra compacta modulates both the direct and indirect pathways, affecting movement.
What is the role of dopamine in the basal ganglia motor loop? (2)

Dopamine facilitates the direct pathway, promoting movement.
Dopamine inhibits the indirect pathway, further supporting movement.
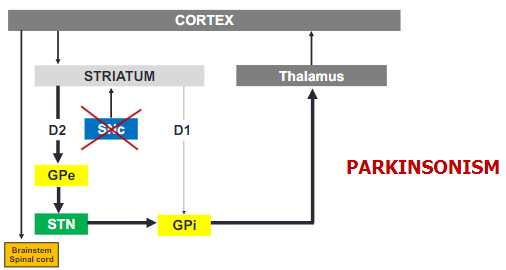
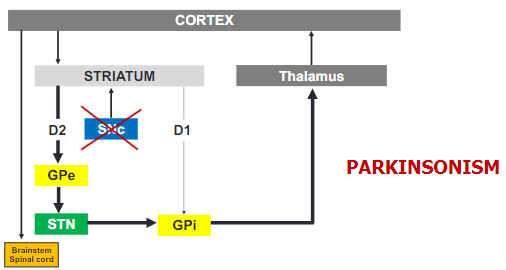
How does the loss of dopamine affect motor function in Parkinson's disease? (3)

The loss of dopamine leads to increased activity in the GPi/SNr.
This increased activity results in the inhibition of thalamocortical pathways.
The outcome is a reduction in motor function, contributing to the symptoms of Parkinson's disease.
What are the key structures involved in the basal ganglia motor loop and their roles? (5)
Cortex: Sends excitatory input (glutamate) to the striatum.
Striatum: Receives input from the cortex and is involved in modulating movement through the direct and indirect pathways.
Thalamus: Relays motor information to the cortex, influenced by the output from the basal ganglia.
GPi: The globus pallidus internal is a critical output nucleus, inhibiting thalamic activity to modulate movement.
GPe: The globus pallidus external modulates the indirect pathway, influencing movement inhibition.
What are the roles of D1 and D2 receptors in the basal ganglia motor loop? (2)
D1 receptors: Facilitate the direct "Go" pathway, promoting movement.
D2 receptors: Facilitate the indirect "Stop" pathway, inhibiting movement.
How does Parkinsonism affect the basal ganglia circuit? (2)
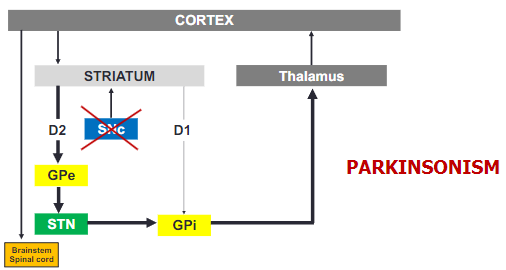
In Parkinsonism, the loss of dopamine causes a shift in basal ganglia activity, leading to overactivity in the GPi.
This increased activity in the GPi results in greater inhibition of the thalamus, impairing motor function.
What is the impact of reduced output from motor areas in Parkinson's disease? (2)
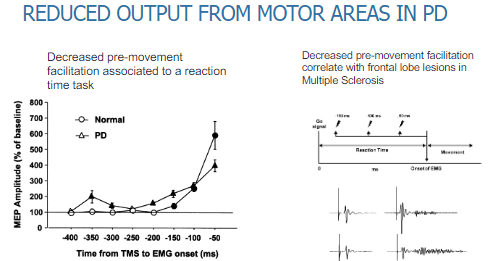
There is decreased pre-movement facilitation, which affects reaction time tasks.
This results in slower initiation of movement and delayed responses during tasks requiring motor action.
How does decreased pre-movement facilitation correlate with other conditions? (2)
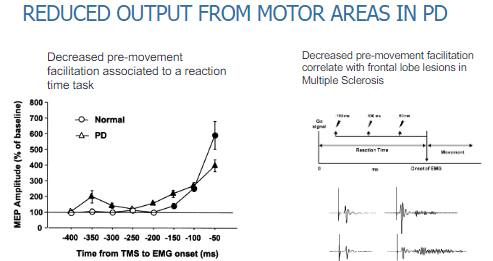
Decreased pre-movement facilitation in Parkinson's disease is similar to the effects seen in frontal lobe lesions.
This type of dysfunction is also observed in Multiple Sclerosis (MS), where frontal lobe damage impairs motor preparation.
What is the effect of Parkinson's disease on medial frontal areas during a free-choice task? (2)
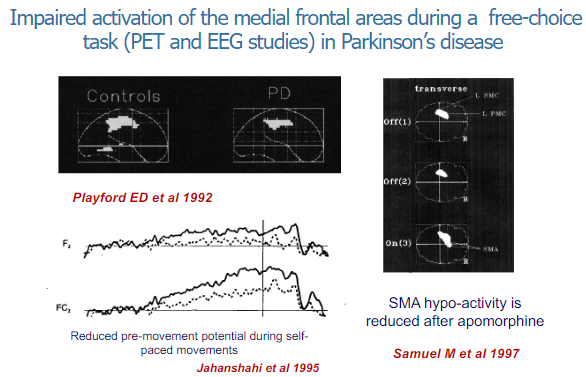
In Parkinson's disease, there is impaired activation of the medial frontal areas during free-choice tasks.
This has been observed through PET (Positron Emission Tomography) and EEG (Electroencephalogram) studies, indicating difficulties in initiating or selecting actions independently.
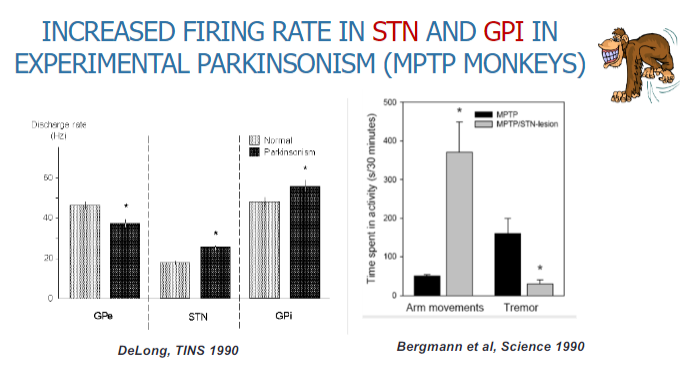
Picture demonstrating increased firing rate in STN and GP in experimental parkinsonism (MPTP monkeys):
How is basal ganglia activity recorded in people with movement disorders during surgery? (2)
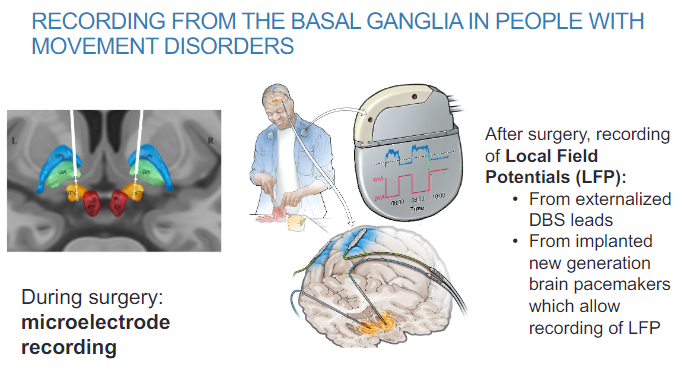
Microelectrode recording is used to measure basal ganglia activity during surgery.
This technique allows for real-time monitoring of neural activity during surgical procedures.
How is basal ganglia activity recorded after surgery in movement disorder patients? (2)
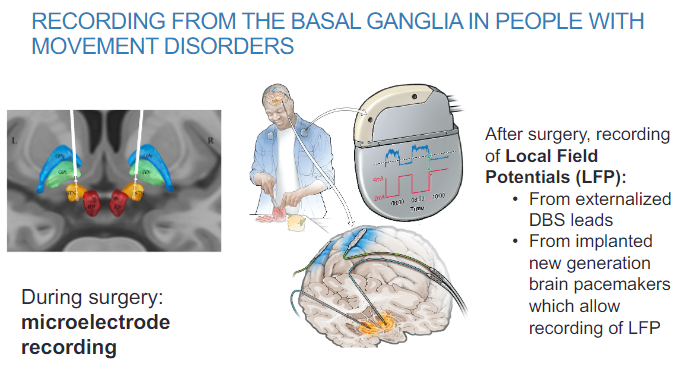
Local Field Potentials (LFP) can be recorded from externalized DBS (Deep Brain Stimulation) leads.
New generation brain pacemakers implanted in patients also allow for LFP recording, providing continuous monitoring of basal ganglia activity.
What is observed in the subthalamic nucleus (STN) of Parkinson's disease patients during recording? (2)
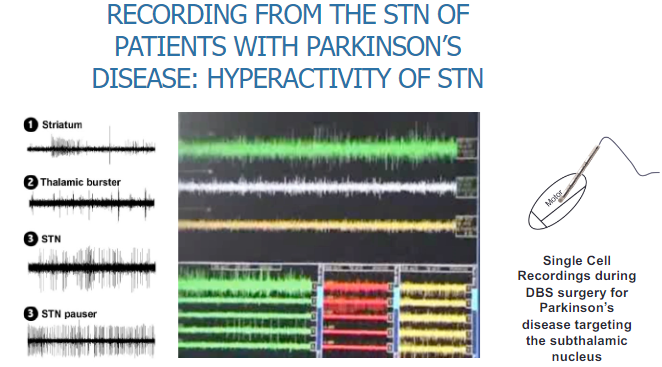
Hyperactivity of the STN is observed in Parkinson’s disease patients.
Single-cell recordings are conducted during DBS (Deep Brain Stimulation) surgery, specifically targeting the subthalamic nucleus, to measure this hyperactivity.
What is chorea and how does the basal ganglia rate model explain it? (3)
Chorea is a hyperkinetic movement disorder characterized by involuntary, jerky movements.
The basal ganglia rate model helps explain the excess of movement in chorea.
This excess movement results from a disruption in the balance between the direct and indirect pathways, leading to reduced inhibition of the thalamus and excessive motor activity.
Why is the rate model of the basal ganglia not enough to explain its physiology and motor control? (4)
The basal ganglia include interconnected pathways beyond the direct and indirect pathways.
The organization is more complex, making it difficult to predict how inputs are transformed to generate outputs.
The model doesn't account for the full range of interactions within the basal ganglia that affect motor control.
The paradox of stereotaxic surgery in Parkinson's disease highlights limitations of the model:
Thalamic lesions do not cause parkinsonism.
GPi lesions do not induce but can ameliorate dyskinesia.
What paradoxes are observed in stereotaxic surgery for Parkinson’s disease? (3)
Thalamic lesions do not cause parkinsonism, challenging the assumption that the thalamus is the sole site for motor dysfunction in Parkinson’s.
GPi lesions do not induce parkinsonism but can reduce dyskinesia, suggesting a more complex relationship in basal ganglia function.
GPi DBS (Deep Brain Stimulation) is effective in treating both dyskinesia and hypokinesia, further complicating the understanding of basal ganglia roles.
What are the problems with the rate model of the basal ganglia based on its anatomy? (3)
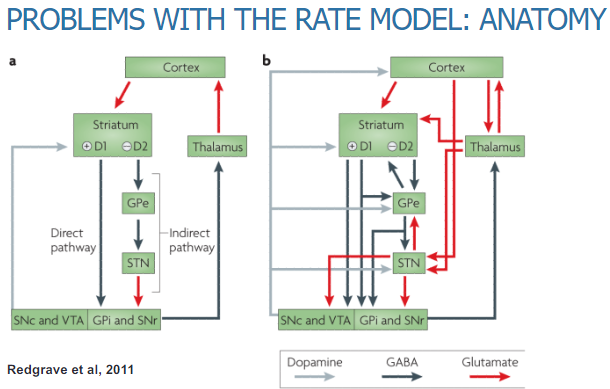
The rate model oversimplifies the anatomy by focusing only on the direct and indirect pathways, neglecting other interconnected pathways within the basal ganglia.
The basal ganglia have a more complex anatomical organization, making it difficult to predict how different regions interact and influence motor control.
The model does not fully account for the intricate anatomy of basal ganglia nuclei and their diverse functions in motor and non-motor control.
Why is the pattern of basal ganglia output important in addition to its level? (2)
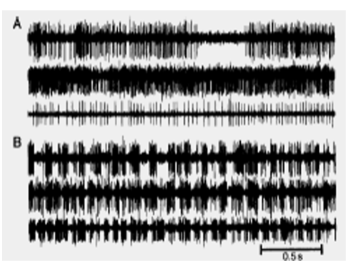
The pattern of basal ganglia output, rather than just its level, plays a crucial role in motor control.
Bergman (1998) demonstrated that microelectrode recordings from single neurons in the GPi of normal and MPTP-treated monkeys showed different firing patterns, suggesting that the timing and rhythm of activity are important for motor function.
What is the role of excessive synchrony in Parkinson's disease? (3)
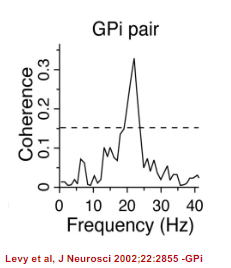
In Parkinson's disease, the discharge of neurons becomes excessively synchronized, leading to abnormal motor control.
Synchronization between pairs of neurons occurs in two principal frequency bands within the basal ganglia of parkinsonian animals and patients:
Low frequencies, possibly related to tremor (more prominent in MPTP-induced monkeys).
13-30 Hz (beta band), which is often associated with motor dysfunction in Parkinson’s disease.
What is "The Oscillation Model" of the basal ganglia, and what insights have been gained from Local Field Potential (LFP) recordings? (3)
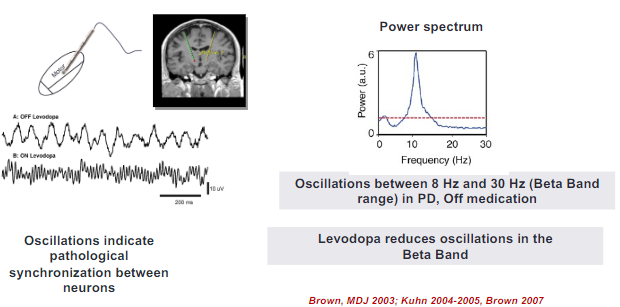
The "Oscillation Model" suggests that the basal ganglia function through synchronized oscillatory activity, which plays a critical role in motor control.
LFP recordings provide insights into how oscillatory activity within the basal ganglia, particularly in the beta band, correlates with motor symptoms in disorders like Parkinson’s disease.
These recordings have revealed that pathological oscillations, such as excessive beta-band activity, contribute to motor dysfunctions like rigidity and bradykinesia in Parkinson's disease.
What are the clinical correlates of abnormal oscillations in the basal ganglia, including beta, alpha, theta, and gamma frequencies? (5)
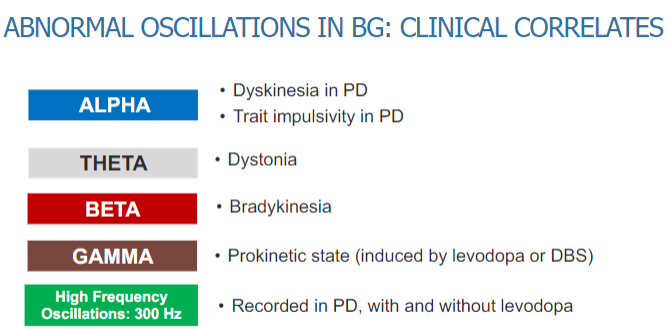
Beta oscillations: Associated with dyskinesia and trait impulsivity in Parkinson’s disease (PD), as well as bradykinesia.
Alpha oscillations: Linked to dystonia, a movement disorder characterized by muscle contractions.
Theta oscillations: Seen in various motor dysfunctions and cognitive impairments in PD.
Gamma oscillations: Associated with motor control and may play a role in dystonia and bradykinesia.
High-frequency oscillations (300 Hz): Recorded in PD patients, both with and without levodopa, and may be related to dyskinesia and other motor symptoms.
How does "The Oscillation Model" explain the relationship between the basal ganglia and motor cortex? (2)
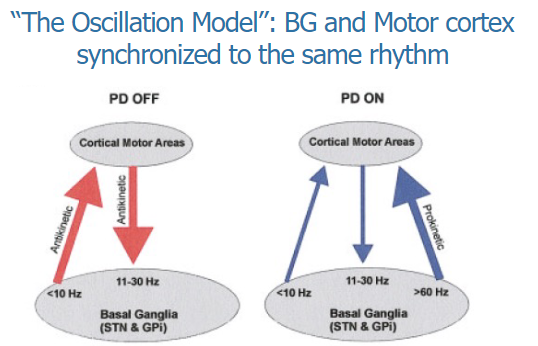
The Oscillation Model suggests that the basal ganglia and motor cortex are synchronized to the same rhythm, which is crucial for coordinated motor control.
This synchronization ensures that motor commands from the motor cortex are effectively processed and executed by the basal ganglia, allowing smooth and controlled movement.
How does beta suppression with levodopa correlate with therapeutic outcomes in Parkinson's disease? (2)
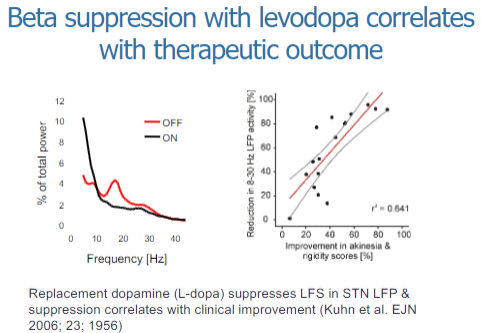
Beta suppression with levodopa is associated with improvements in motor symptoms in Parkinson's disease.
The degree of beta suppression correlates with the therapeutic outcome, meaning greater suppression of beta oscillations leads to better motor control and symptom relief.
How does deep brain stimulation (DBS) of the basal ganglia improve motor control in Parkinson’s disease? (2)
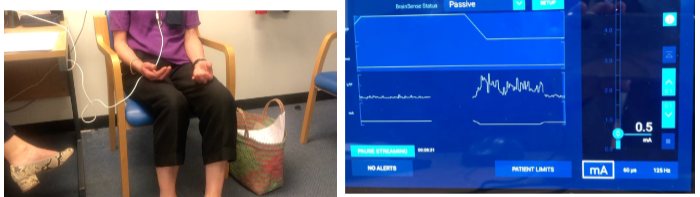
Deep brain stimulation (DBS) of the basal ganglia helps improve motor control in Parkinson's disease by modulating abnormal neural activity.
DBS leads to the suppression of beta oscillations, which are linked to motor dysfunction, contributing to improved motor function and symptom management.
How does suppressing beta power improve bradykinesia in Parkinson’s disease, and what effect does direct stimulation of the STN at beta band frequencies have? (3)
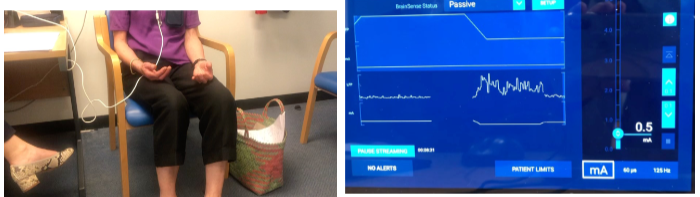
Suppressing beta power improves bradykinesia in Parkinson's disease by reducing the pathological oscillations associated with motor dysfunction, leading to better movement control.
Recordings from new-generation DBS pacemakers show that reducing beta power can result in significant improvements in motor symptoms, including bradykinesia.
However, direct stimulation of the subthalamic nucleus (STN) at beta band frequencies can worsen parkinsonism, suggesting that the timing and frequency of stimulation are critical for therapeutic outcomes.
How does synchronization in the beta band limit motor processing? (2)
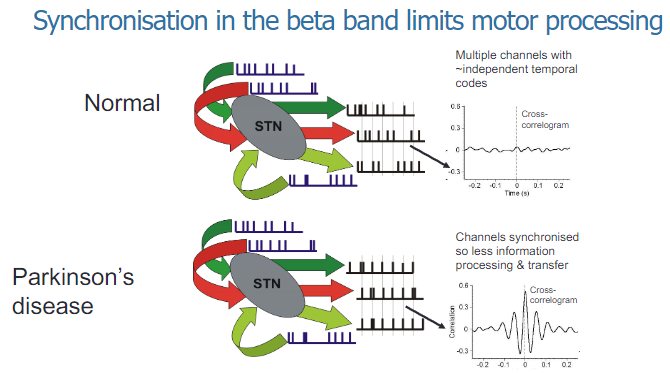
Synchronization in the beta band within the basal ganglia is associated with a reduction in motor processing, leading to motor dysfunction such as bradykinesia in Parkinson's disease.
Excessive beta-band synchronization impairs the ability of the motor system to process and execute smooth, coordinated movements.
What is required to move or not move, according to the model of sensory states and motor control? (3)
To move, there must be a change from one stable sensory state to another stable sensory state.
Turning down the current sensory state (lower beta power) is necessary to initiate movement.
Accurate prediction of the new sensory state is needed to guide the movement, followed by a mechanism to stabilize the new sensory state (higher beta power) to maintain control over the movement.
How does beta power relate to sensory states and movement? (2)
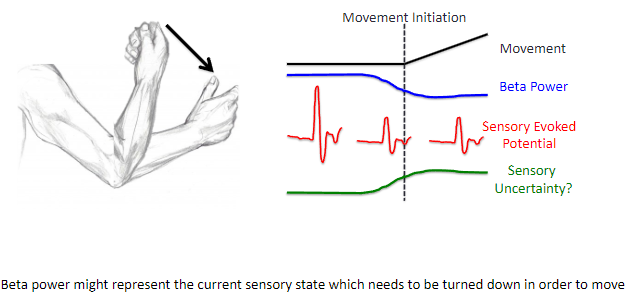
Beta power might represent the current sensory state, which needs to be turned down to initiate movement.
Reducing beta power helps transition from the current stable sensory state to a new state, enabling movement to occur.
What is the free-energy principle, and how does it relate to brain function? (2)
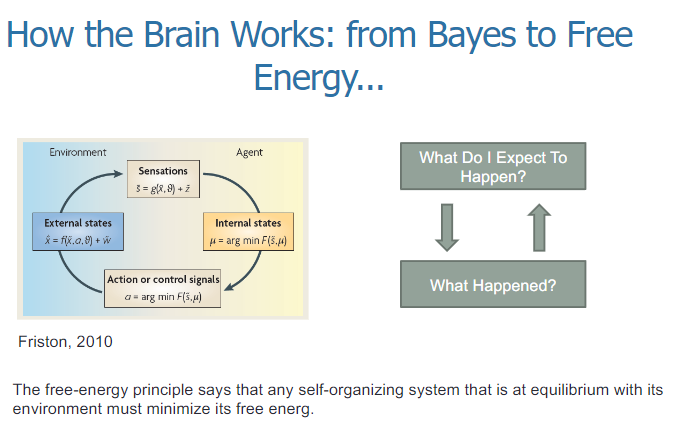
The free-energy principle states that any self-organizing system at equilibrium with its environment must minimize its free energy.
In the context of the brain, this principle suggests that the brain constantly makes predictions about what will happen (expectations) and updates its beliefs based on the difference between what was expected and what actually happened (prediction error) to reduce free energy.
What are the functions of the cerebellum, parietal cortex, basal ganglia, and motor cortices in motor control? (4)
Cerebellum: Functions in system identification by building internal models to predict sensory outcomes of motor commands and correct motor commands through internal feedback.
Parietal Cortex: Responsible for state estimation by integrating predicted proprioceptive and visual outcomes with sensory feedback to form a belief about how the commands affected the body and environment.
Basal Ganglia: Involved in optimal control by learning costs and rewards associated with sensory states and estimating the "cost-to-go" during motor task execution.
Primary and Premotor Cortices: Implement the optimal control policy by transforming beliefs about proprioceptive and visual states into motor commands.
What is the role of the primary motor cortex (M1) beyond effectors? (4)
The classic homunculus in the primary motor cortex (M1) is interrupted by regions with distinct connectivity, structure, and function, alternating with effector-specific areas (e.g., foot, hand, and mouth).
These inter-effector regions have strong functional connectivity to each other and to the cingulo-opercular network (CON).
The inter-effectors lack movement specificity and co-activate during action planning, such as coordinating hands and feet or axial body movement (e.g., abdomen or eyebrows).
M1 is punctuated by a system for whole-body action planning, called the somato-cognitive action network (SCAN).
A picture demonstrating a system approach to motor control:
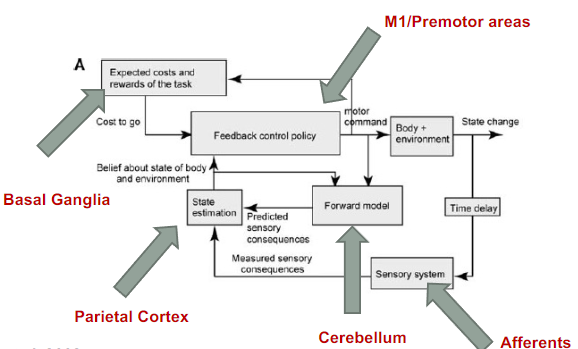
What are the basal ganglia, movement disorders, and efference copy? (3)
Basal Ganglia: A group of nuclei that are central to the control of movement.
Movement Disorders: A diverse array of neurological illnesses that result in disordered control of movement.
Efference Copy: A prediction of the expected movement outcome generated at the same time as the movement signal, which is then compared with the actual movement outcome.