How are fatty acids absorbed and transported?
1. Triacylglycerides are emulsified by bile salts in the small intestine to form micelles
2. Intestinal lipases degrade micelles into mono/diglycerides and free fatty acids
3. fatty acids diffuse across epithelial cells of the small inestine and are converted back into triacylglycerides
4. Triacylglycerides are packed with cholesterol and apolipoproteins in aggregates called chylomicrons for transport
5. Chylomicrons move through blood and lymph systems to target tissues, picking up ApoC-II (alipoprotein) from high density lipoproteins along with it
6. ApoC-II activates lipoprotein lipase in cells which breaks down the chylomicron into free fatty acids and monacylglycerides
7. fatty acids enter the cell
8. fatty acids in muscle cells are oxidized for energy while fatty acids in adipose tissue are reesterified for storage
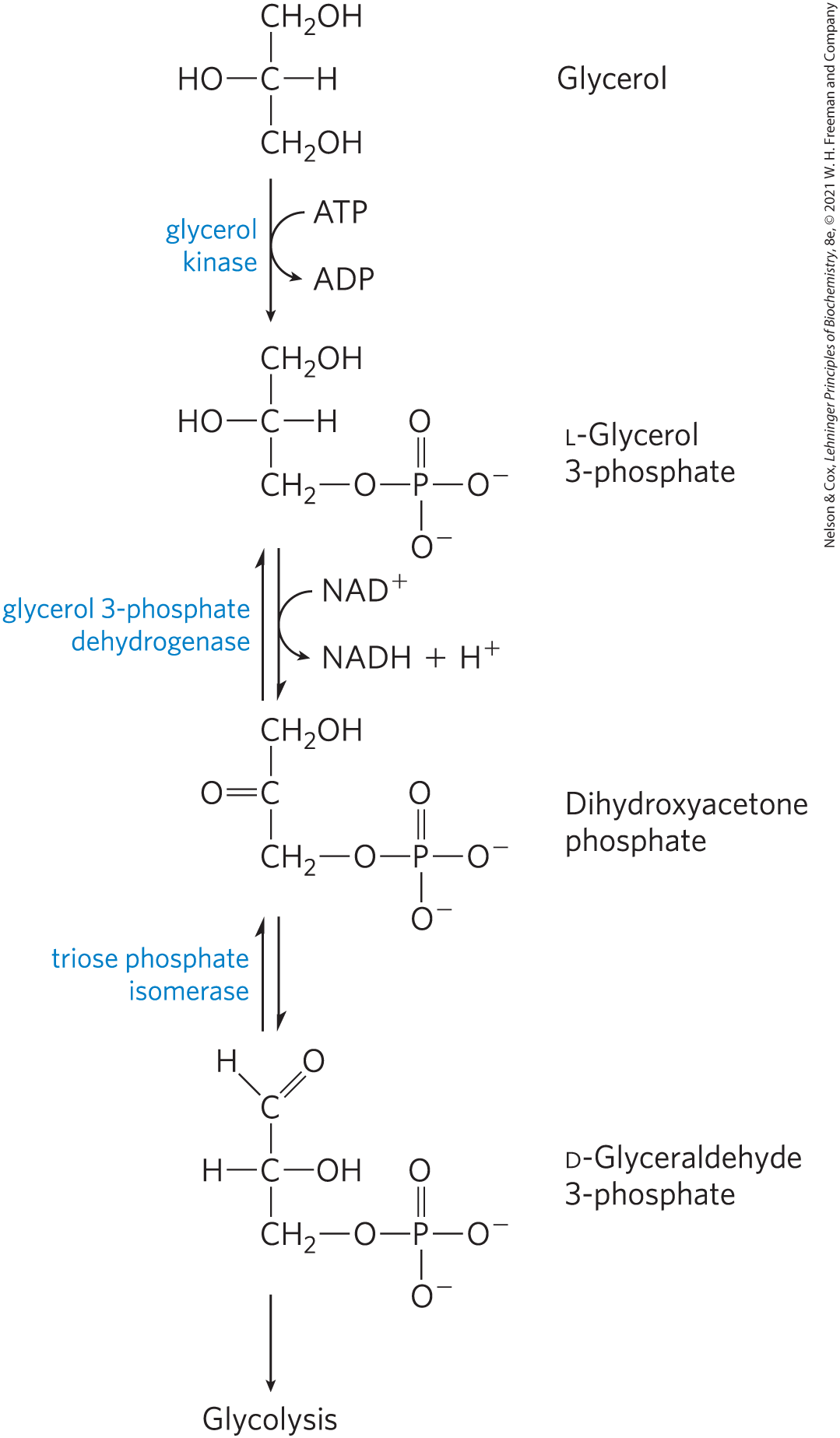
Write the reactions for glycerol to D-glyceraldehyde 3-P
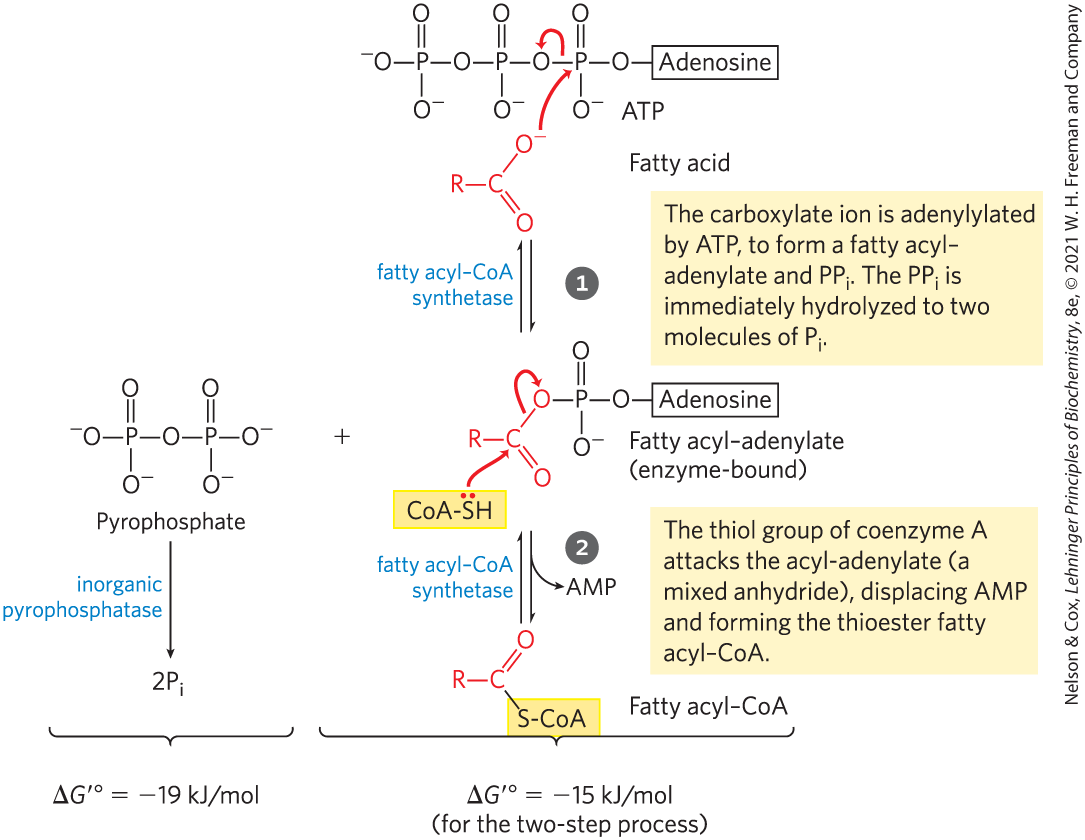
Write the reactions for the activation of a fatty acyl-CoA
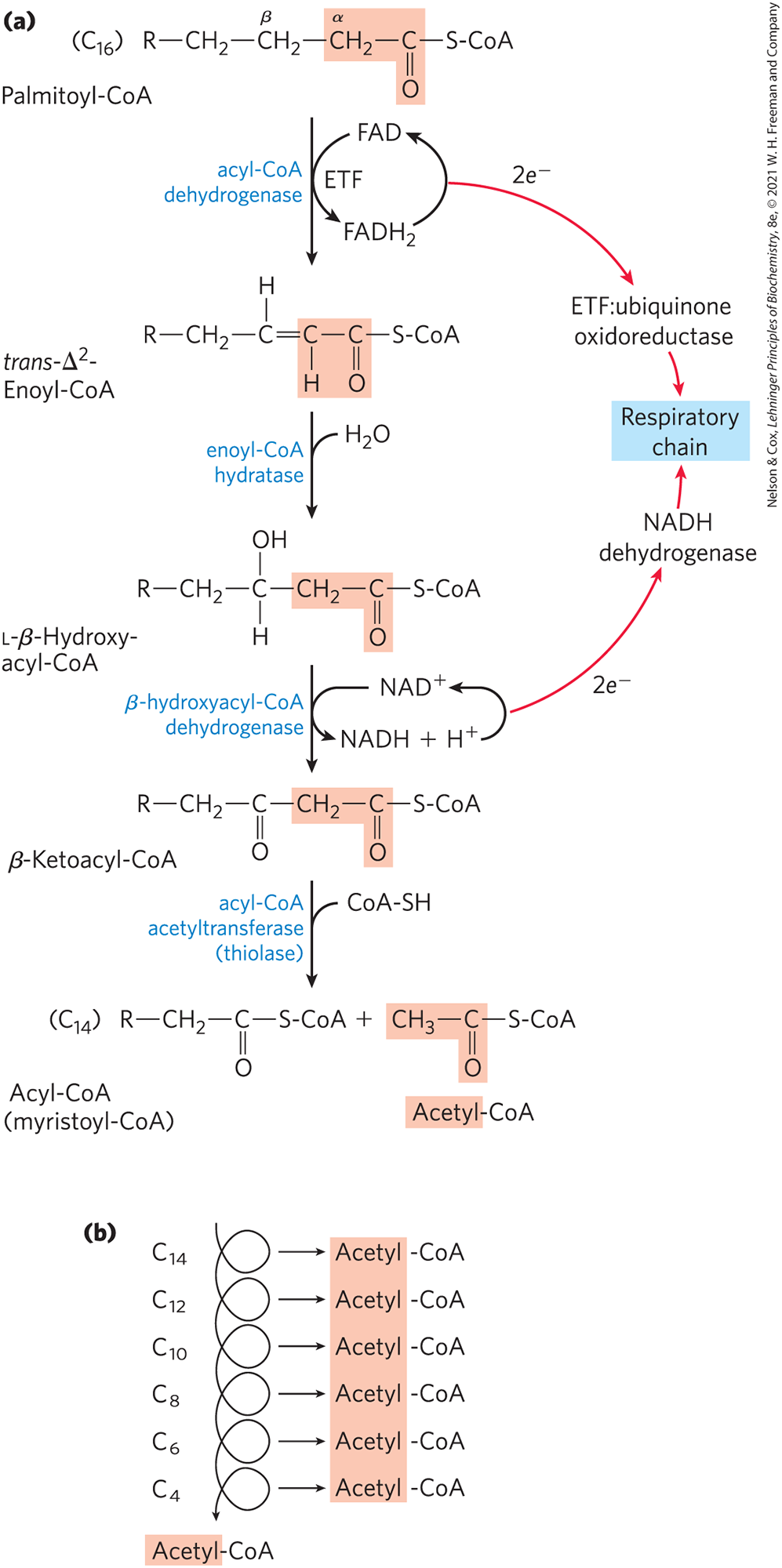
Write the reactions for B-Oxidation pathway
Describe the acyl-carnitine/carnitine pathway
1. Fatty acyl-CoA molecules are acted upon by the enzyme CAT1 on the outer mitochondrial membrane to displace the CoA with a carnitine molecule
2. The molecule then disfuses across the outer-membrane to the inter-membrane space where it then must be transported with a carnitine pump.
3. The carnitine pump lets one fatty acyl/carnitine molecule into the matrix while simultaneously releasing one carnitine molecule
4. The enzyme CAT2 acts upon the fatty-acyl/carnitine molecule in the matrix to displace carnitine with coA, releasing the original molecule into the matrix and expelling a carnitine molecule back to the outer membrane to be used again.
Why does the acyl/carnitine pathway exist? Why can't fatty acids transport directly into the matrix?
1. Long chain fatty acids (higher than 14 carbons) cannot freely diffuse unlike smaller fatty acids which are less hydrophobic. Attaching a carnitine molecule makes it more energetically favorable to diffuse across the membrane.
2. This method links the 2 separate pools of coA and of fatty acyl-coA, one in the cytosol which is used for synthesis of fatty acids and the other in the matrix used for oxidative degradation of pyruvate, fatty acids, and amino acids
3. carnitine-mediated entry process is the rate-limiting step for oxidation of fatty acids in the mitochondria. CAT1 is limited by malonyl-CoA which is the first intermediate in fatty acid synthesis, which inhibits the simultaneous synthesis and degradation of fatty acids.
How many ATP can ultimately be generated from a triacylglycerol?
4 ATP are formed with each pass of beta oxidation. Palmitoyl coA a 16 carbon molecule goes through 7 passes (2C are left for acetyl coA), so 28 ATP are generated. 8 Acetyl coA are ultimately generated, which can make 10 ATP. So from palmitoyl coA, 80 more ATP are made, making 108 total ATP produced. Now the energetic cost of activating a fatty acid requires 2ATP, so a net ATP yield from Palmitoyl would be 106 ATP
Be familiar with the general rationalization of oxidation of monounsaturated and polyunsaturated fatty acids. If you are familiar with the enzymes, you will be able to answer any question about the B-oxidation pathway from above.
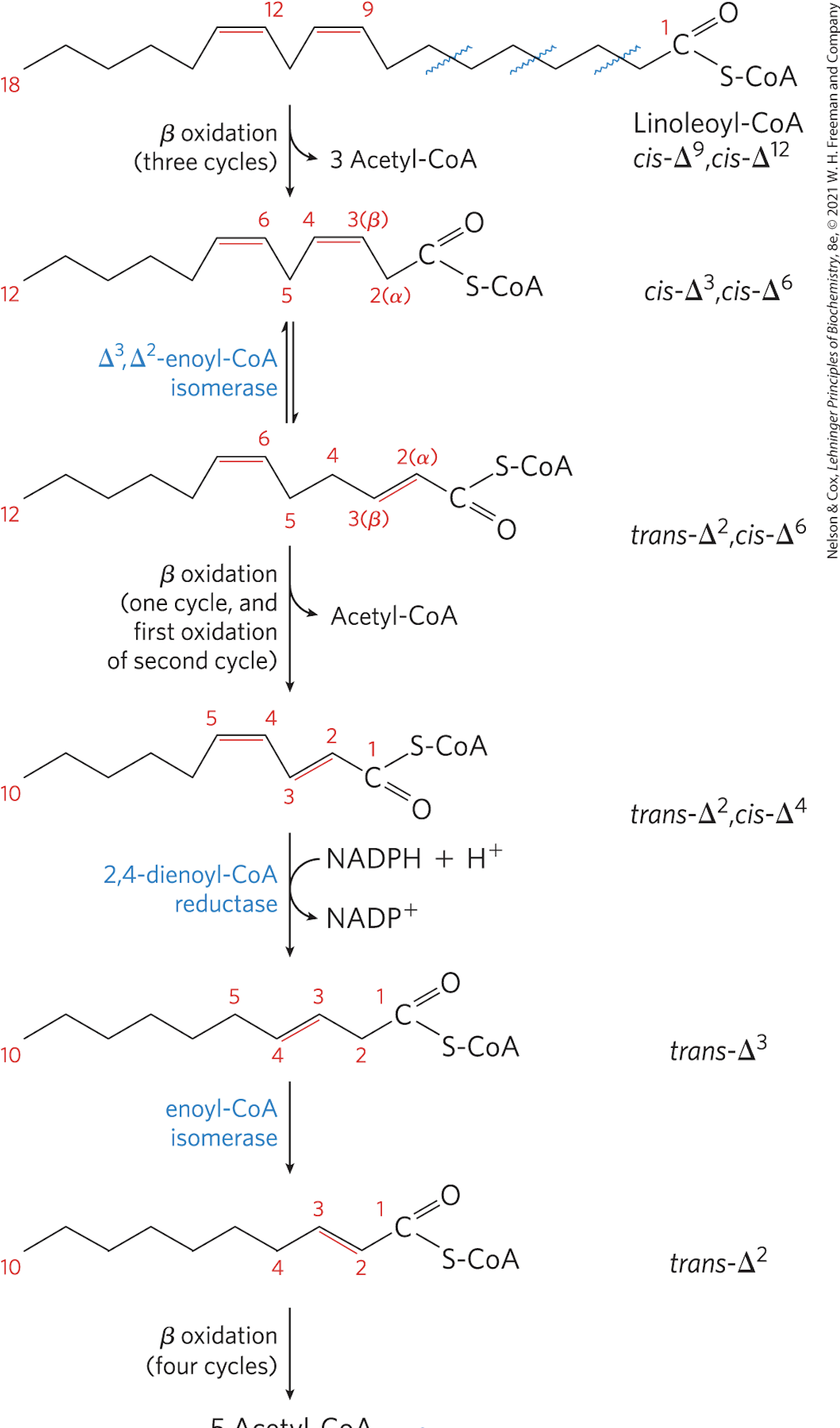
1. Isomerase. When a fatty acyl has a double bond anywhere, it will go through the process of beta oxidation until it reaches the double bond at the 𝚫3 position. From here the enzyme 𝚫3, 𝚫2 enoyl-CoA isomerase will isomerize the molecule from a cis-𝚫3 position into a trans 𝚫2 position to be acted upon by the next step in beta oxidation, trans 𝚫2 enoyl-coA hydratase.
2. Reductase. Polyunsaturated fatty acids need another enzyme to go through all of beta oxidation. The first double bond is acted upon by the isomerase, but the next is acted upon by 2,4 dienoyl-CoA reductase which uses NADP+ to convert the molecule into a useable form.
What is the difference between mitochondria and peroxisome function?
1. Peroxisomes of animal tissues specialize in the oxidation of very long chain fatty acids and branched fatty acids. A oxidation is used to convert these into products to be used for B oxidation
2. In the first step of B oxidation in a peroxisome, the flavoprotein acyl-oxidase that introduces the double bond also passes electrons directly to O2, producing H2O2 which must be cleaved by a catalase. The energy released in the first step dissipates as heat.
3. The NADH formed in the second step cannot be reoxidized in the peroxisome, so reducing equivalents are exported to the cytosol to enter mitochondria. Acetyl-CoA is also transported as a biosynthesis precursor.
What are ketone bodies and how do they arise?
Ketone bodies are formed in the liver from Acetyl-CoA and are transported to extrahepatic tissue and are converted to Acetyl-CoA for the citric acid cycle. thiolase condenses 2 acetyl-coA molecules. During starvation, ketone bodies are produced as an energy source for the brain, they are never consumed by the liver but are by all other tissues.