Bronsted-Lowry
acid is a proton (H+) donor; base is a proton acceptor
Lewis
acid is an electron pair acceptor; base is an electron pair donor
Pyrrolidines, 9-10
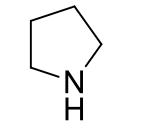
name the heterocycle & give the pKa
piperidines, 9-10
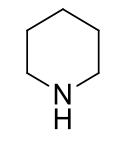
name the heterocycle & give the pKa
piperazines, 9-10
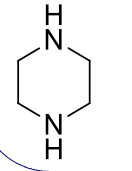
name the heterocycle & give the pKa
Morpholines, 9-10
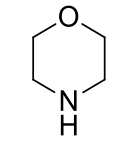
name the heterocycle & give the pKa
tetrahydroisoquinolines, 9-10
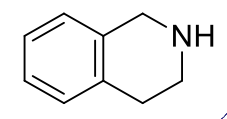
name the heterocycle & give the pKa
anilines, 4-5
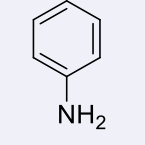
name the heterocycle & give the pKa
pyridines, 4-5
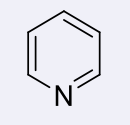
name the heterocycle & give the pKa
quinolines, 4-5
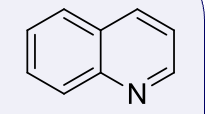
name the heterocycle & give the pKa
benzimidazoles, 4-5
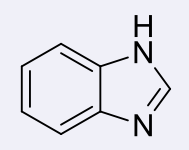
name the heterocycle & give the pKa
isoquinolines, 4-5
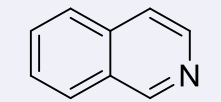
name the heterocycle & give the pKa
pyrazoles, 2-3
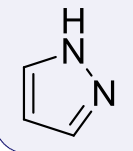
name the heterocycle & give the pKa
thiazoles, 2-3
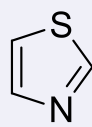
name the heterocycle & give the pKa
pyridazines, 2-3
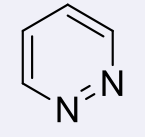
name the heterocycle & give the pKa
imidazoles, 6-7
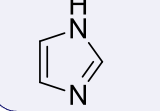
name the heterocycle & give the pKa
bisaniline, neutral
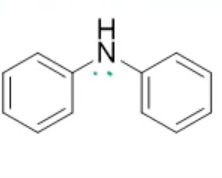
name the heterocycle & give the pKa
indole, neutral
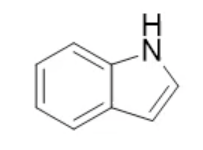
name the heterocycle & give the pka
phenothiazine, neutral
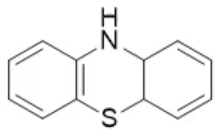
name the heterocycle & give the pka
pyrazine, neutral
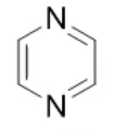
name the heterocycle & give the pka
oxazole, neutral
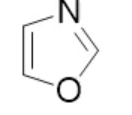
name the heterocycle & give the pka
pyrrole, neutral
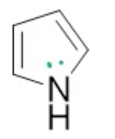
name the heterocycle & give the pka
benzoxazole, neutral
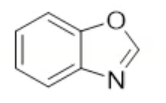
name the heterocycle & give the pka
benzthiazole, neutral
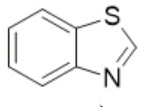
name the heterocycle & give the pka