Imperfections in Solid Materials
advertisement

Imperfections in Solid
Materials
R. Lindeke
ENGR 2110
In our pervious Lecture when
discussing Crystals we
ASSUMED PERFECT ORDER
In real materials we find:
Crystalline Defects or lattice irregularity
Most real materials have one or more “errors in perfection”
with dimensions on the order of an atomic diameter to many
lattice sites
Defects can be classification:
1. according to geometry
(point, line or plane)
2. dimensions of the defect
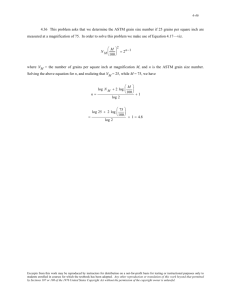
ISSUES TO ADDRESS...
• What are the solidification mechanisms?
• What types of defects arise in solids?
• Can the number and type of defects be varied
and controlled?
• How do defects affect material properties?
• Are defects always undesirable?
Imperfections in Solids
• Solidification- result of casting of molten material
– 2 steps
• Nuclei form
• Nuclei grow to form crystals – grain structure
• Start with a molten material – all liquid
nuclei
liquid
crystals growing
grain structure
Adapted from Fig.4.14 (b), Callister 7e.
• Crystals grow until they meet each other
Polycrystalline Materials
Grain Boundaries
• regions between crystals
• transition from lattice of
one region to that of the
other
• ‘slightly’ disordered
• low density in grain
boundaries
– high mobility
– high diffusivity
– high chemical reactivity
Adapted from Fig. 4.7, Callister 7e.
Solidification
Grains can be - equiaxed (roughly same size in all directions)
- columnar (elongated grains)
~ 8 cm
heat
flow
Columnar in
area with less
undercooling
Shell of
equiaxed grains
due to rapid
cooling (greater
T) near wall
Adapted from Fig. 4.12, Callister 7e.
Grain Refiner - added to make smaller, more uniform, equiaxed grains.
Point Defects
• Vacancies:
-vacant atomic sites in a structure.
Vacancy
distortion
of planes
• Self-Interstitials:
-"extra" atoms positioned between atomic sites.
selfinterstitial
distortion
of planes
SELF-INTERSTITIAL: very rare occurrence
• This defect occurs when an atom from the crystal occupies
the small void space (interstitial site) that under
ordinary circumstances is not occupied.
• In metals, a self-interstitial introduces relatively (very!)
large distortions in the surrounding lattice.
POINT DEFECTS
• The simplest of the point defect is a vacancy, or vacant lattice site.
• All crystalline solids contain vacancies.
• Principles of thermodynamics is used explain the necessity of the
existence of vacancies in crystalline solids.
• The presence of vacancies increases the entropy (randomness) of
the crystal.
• The equilibrium number of vacancies for a given quantity of
material depends on and increases with temperature as
follows:
Total no. of atomic sites
Energy required to form vacancy
Equilibrium no. of vacancies
Nv= N exp(-Qv/kT)
T = absolute temperature in Kelvin
k = gas or Boltzmann’s constant
Measuring Activation Energy
-Q
Nv
v
exp
=
kT
N
• We can get Qv from
an experiment.
• Measure this...
• Replot it...
Nv
ln
N
Nv
N
note:
N
N A El
AEl
slope
-Qv /k
exponential
dependence!
T
defect concentration
1/T
Example Problem 4.1
Calculate the equilibrium number of vacancies per cubic meter for copper at
1000°C. The energy for vacancy formation is 0.9 eV/atom; the atomic weight and
density (at 1000 ° C) for copper are 63.5 g/mol and 8.4 g/cm3, respectively.
Solution.
Use equation 4.1. Find the value of N, number of atomic sites per cubic meter for
copper, from its atomic weight Acu, its density, and Avogadro’s number NA.
N A (6.023x10 23 atoms / mol)(8.4 g / cm3 )(106 cm3 / m3 )
N
ACu
63.5 g / mol
8.0x10 28 atoms / m3
Thus, the number of vacancies at 1000 C (1273K ) ie equal to
Continuing:
Qv
N v N exp
kT
(8.0x10
28
(
0
.
9
eV
atoms / m ) exp
(8.62 x10-5 eV / K )(1273K )
3
2.2x10 25 vacancies /m 3
And Note: for MOST MATERIALS just below
Tm Nv/N = 10-4
Here: 0.0022/8 = .000275 = 2.75*10-4
Point Defects in Alloys
Two outcomes if impurity (B) added to host (A):
• Solid solution of B in A (i.e., random dist. of point defects)
OR
Substitutional solid soln.
(e.g., Cu in Ni)
Interstitial solid soln.
(e.g., C in Fe)
• Solid solution of B in A plus particles of a new
phase (usually for a larger amount of B)
Second phase particle
--different composition
--often different structure.
Imperfections in Solids
Conditions for substitutional solid solution (S.S.)
• Hume – Rothery rules
– 1. r (atomic radius) < 15%
– 2. Proximity in periodic table
• i.e., similar electronegativities
– 3. Same crystal structure for pure metals
– 4. Valency equality
• All else being equal, a metal will have a greater tendency
to dissolve a metal of higher valency than one of lower
valency (it provides more electrons to the “cloud”)
Imperfections in Solids
Application of Hume–Rothery rules – Solid
Solutions
Element
Atomic Crystal
ElectroRadius Structure
(nm)
1. Would you predict
more Al or Ag
to dissolve in Zn?
More Al because size is closer and val. Is
higher – but not too much – FCC in HCP
2. More Zn or Al
in Cu?
Surely Zn since size is closer thus
causing lower distortion (4% vs 12%)
Cu
C
H
O
Ag
Al
Co
Cr
Fe
Ni
Pd
Zn
0.1278
0.071
0.046
0.060
0.1445
0.1431
0.1253
0.1249
0.1241
0.1246
0.1376
0.1332
Valence
negativity
FCC
1.9
+2
FCC
FCC
HCP
BCC
BCC
FCC
FCC
HCP
1.9
1.5
1.8
1.6
1.8
1.8
2.2
1.6
+1
+3
+2
+3
+2
+2
+2
+2
Table on p. 106, Callister 7e.
Imperfections in Solids
• Specification of composition
– weight percent
m1
C1
x 100
m1 m2
m1 = mass of component 1
– atom percent
nm1
C
x 100
n m1 n m 2
'
1
nm1 = number of moles of component 1
Wt. % and At. % -- An example
Typically we work with a basis of 100g or 1000g
given: by weight -- 60% Cu, 40% Ni alloy
600 g
nCu
9.44m
63.55 g / m
400 g
nNi
6.82m
58.69 g / m
9.44
'
CCu
.581 or 58.1%
9.44 6.82
6.82
'
CNi
.419 or 41.9%
9.44 6.82
Converting Between: (Wt% and At%)
C1 A2
C
100
C1 A2 C2 A1
'
1
C2 A1
C
100
C1 A2 C2 A1
'
2
C A1
C1 '
100
'
C1 A1 C2 A2
Converts from
wt% to At% (Ai is
atomic weight)
'
1
C A2
C2 '
100
'
C1 A1 C2 A2
'
2
Converts from
at% to wt% (Ai is
atomic weight)
Determining Mass of a Species per Volume
C
1
C1"
C1 C2
1
2
C
"
2
C2
C1 C2
1
2
103
103
• i is density of pure
element in g/cc
• Computed this way,
gives “concentration”
of speciesi in kg/m3 of
the bulk mixture
(alloy)
Line Defects
Are called Dislocations:
And:
• slip between crystal planes result when dislocations move,
• this motion produces permanent (plastic) deformation.
Schematic of Zinc (HCP):
• before deformation
• after tensile elongation
Adapted from Fig. 7.8, Callister 7e.
slip steps which are
the physical
evidence of large
numbers of
dislocations
slipping along the
close packed plane
{0001}
Linear Defects (Dislocations)
– Are one-dimensional defects around which atoms are
misaligned
• Edge dislocation:
– extra half-plane of atoms inserted in a crystal structure
– b (the berger’s vector) is (perpendicular) to dislocation
line
• Screw dislocation:
– spiral planar ramp resulting from shear deformation
– b is (parallel) to dislocation line
Burger’s vector, b: is a measure of lattice distortion and is
measured as a distance along the close packed directions in
the lattice
Edge Dislocation
Edge Dislocation
Fig. 4.3, Callister 7e.
Motion of Edge Dislocation
• Dislocation motion requires the successive bumping
of a half plane of atoms (from left to right here).
• Bonds across the slipping planes are broken and
remade in succession.
http://www.wiley.com/
college/callister/CL_E
WSTU01031_S/vmse
/dislocations.htm
Atomic view of edge
dislocation motion from
left to right as a crystal
is sheared.
(Courtesy P.M. Anderson)
Screw Dislocations
b
Dislocation
line
Burgers vector b
(b)
(a)
Adapted from Fig. 4.4, Callister 7e.
Edge, Screw, and Mixed Dislocations
Mixed
Edge
Adapted from Fig. 4.5, Callister 7e.
Screw
Imperfections in Solids
Dislocations are visible in (T) electron micrographs
Adapted from Fig. 4.6, Callister 7e.
Dislocations & Crystal Structures
• Structure: close-packed
planes & directions
are preferred.
close-packed plane (bottom)
view onto two
close-packed
planes.
close-packed directions
close-packed plane (top)
• Comparison among crystal structures:
FCC: many close-packed planes/directions;
HCP: only one plane, 3 directions;
BCC: none “super-close” many “near close”
• Specimens that
were tensile
tested.
Mg (HCP)
tensile direction
Al (FCC)
Planar Defects in Solids
• One case is a twin boundary (plane)
– Essentially a reflection of atom positions across the
twinning plane.
Adapted from Fig. 4.9, Callister 7e.
• Stacking faults
– For FCC metals an error in ABCABC packing sequence
– Ex: ABCABABC
MICROSCOPIC EXAMINATION
Applications
• To Examine the structural elements and defects that influence the
properties of materials.
• Ensure that the associations between the properties and structure (and
defects) are properly understood.
• Predict the properties of materials once these relationships have been
established.
Structural elements exist in ‘macroscopic’
and ‘microscopic’ dimensions
MACROSCOPIC EXAMINATION: The shape and average size or
diameter of the grains for a polycrystalline specimen are large
enough to observe with the unaided eye.
Optical Microscopy
• Useful up to 2000X magnification (?).
• Polishing removes surface features (e.g., scratches)
• Etching changes reflectance, depending on crystal
orientation since different Xtal planes have different
reactivity.
crystallographic planes
Adapted from Fig. 4.13(b) and (c), Callister
7e. (Fig. 4.13(c) is courtesy
of J.E. Burke, General Electric Co.
Micrograph of
brass (a Cu-Zn alloy)
0.75mm
Optical Microscopy
Grain boundaries...
• are planer imperfections,
• are more susceptible
to etching,
• may be revealed as
dark lines,
• relate change in crystal
orientation across
boundary.
polished surface
(a)
surface groove
grain boundary
Adapted from Fig. 4.14(a)
and (b), Callister 7e.
(Fig. 4.14(b) is courtesy
of L.C. Smith and C. Brady,
the National Bureau of
Standards, Washington, DC
[now the National Institute of
Standards and Technology,
Gaithersburg, MD].)
ASTM grain
size number
N = 2n-1
number of grains/in2
at 100x
magnification
Fe-Cr alloy
(b)
GRAIN SIZE DETERMINATION
The grain size is often determined when the properties of
a polycrystalline material are under consideration. The
grain size has a significant impact of strength and
response to further processing
Linear Intercept method
• Straight lines are drawn through several
photomicrographs that show the grain
structure.
• The grains intersected by each line segment are
counted
• The line length is then divided by an average
number of grains intersected.
•The average grain diameter is found by dividing this
result by the linear magnification of the
photomicrographs.
ASTM (American Society for testing and Materials)
ASTM has prepared several standard comparison charts, all having different
average grain sizes. To each is assigned a number from 1 to 10, which is termed
the grain size number; the larger this number, the smaller the grains.
VISUAL CHARTS (@100x) each with a number
Quick and easy – used for steel
Grain size no.
No. of grains/square inch
N = 2 n-1
NOTE: The ASTM grain size is related (or relates)
a grain area AT 100x MAGNIFICATION
Determining Grain Size, using a micrograph
taken at 300x
• We count 14 grains
in a 1 in2 area on
the image
• The report ASTM
grain size we need
N at 100x not 300x
• We need a
conversion method!
2
M
n -1
NM
2
100
M is mag. of image
N M is measured grain count at M
now solve for n:
log( N M ) 2 log M - log 100 n - 1 log 2
n
log N m 2 log M - 4
1
log 2
n
log 14 2 log 300 - 4
1 7.98 8
0.301
For this same material, how many Grains
would I expect /in2 at 100x?
N 2
n -1
8 -1
2
128 grains/in
2
Now, how many grain would I expect at 50x?
2
100
100
NM 2
128*
M
50
2
2
N M 128* 2 512 grains/in
8 -1
2
At 100x
Number of Grains/in2
600
500
400
300
200
100
0
0
2
4
6
Grain Size number (n)
8
10
12
Electron Microscopes
beam of electrons of
shorter wave-length
(0.003nm) (when
accelerated across large
voltage drop)
Image formed with
Magnetic lenses
High resolutions and
magnification (up to
50,000x SEM); (TEM up
to 1,000,000x)
Scanning Tunneling Microscopy (STM)
• Uses a moveable Probe of very small diameter
to move over a surface
• Atoms can be arranged and imaged!
Photos produced from
the work of C.P. Lutz,
Zeppenfeld, and D.M.
Eigler. Reprinted with
permission from
International Business
Machines Corporation,
copyright 1995.
Carbon monoxide
molecules arranged
on a platinum (111)
surface.
Iron atoms arranged
on a copper (111)
surface. These Kanji
characters represent
the word “atom”.
Summary
• Point, Line, and Area defects exist in solids.
• The number and type of defects can be varied and
controlled
– T controls vacancy conc.
– amount of plastic deformation controls # of dislocations
– Weight of charge materials determine concentration of
substitutional or interstitial point ‘defects’
• Defects affect material properties (e.g., grain boundaries
control crystal slip).
• Defects may be desirable or undesirable
– e.g., dislocations may be good or bad, depending on whether
plastic deformation is desirable or not.
– Inclusions can be intention for alloy development