Oxygen
advertisement

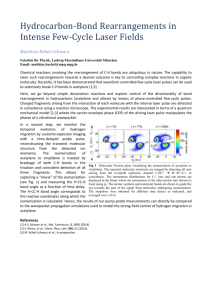
Lesson 1 WS- 01- 4 – 1 Oxy Acetylen Cutting Oxygen Characteristics Technical specification Production Safety It is the colorless, odorless, tasteless gas that supports life and makes combustion possible. It constitutes about one-fifth of the earth’s atmosphere (20.99 percent by volume). It is a transparent, pale blue liquid slightly heavier than water at temperatures ranging below approximately -300° F. All elements except the inert gases combine with oxygen, usually to form oxides. Oxygen Characteristics Technical specification Oxygen purity is vital to efficient production. For example, 99.5 percent pure oxygen can produce high-quality, flame-cut edges. A drop of one percent in oxygen purity slows cutting speed 25 percent. At 95 percent oxygen purity, an oxy-fuel cutting torch will not cut steel at all. Production Safety WEIGHT GAS LIQUID POUNDS Lb KILOGRAMS Kg CUBIC FEET SCF CUBIC METERS Nm3 GALLONS Gal LITERS L 1.0 0.4536 12.076 0.3174 0.1050 0.3977 1 Kilogram 2.205 1.0 26.62 0.6998 0.2316 0.8767 1 SCF Gas 0.08281 0.03756 1.0 0.02628 0.008691 0.0329 1 Nm3 Gas 3.151 1.4291 38.04 1.0 0.3310 1.2528 1 Gal Liquid 9.527 4.322 115.1 3.025 1.0 3.785 1 L Liquid 2.517 1.1417 30.38 0.7983 0.2642 1.0 1 Pound Oxygen Characteristics Technical specification Production Safety • Cylinder oxygen is produced from vaporized liquid oxygen. Its minimum guaranteed purity is 99.5 percent. • Liquid oxygen is classified as Type II by the Compressed Gas Association (CGA). CGA Pamphlet G-4.3 Type II, Grade B and the United States Pharmacopia (USP) specifications are considered standards for "commercial" liquid oxygen. A variety of other CGA oxygen Grades are available on special order. These range from CGA Grade C, also 99.5 percent oxygen content to CGA Grade H (99.995 percent oxygen). Also available is high purity ULSI grade and ultra high purity VLSI grade (99.9999 percent pure) liquid oxygen for customers requiring stringent purity standards. Oxygen Characteristics Technical specification Production Safety •Oxygen makes it much easier for materials to ignite. Even materials that would not normally burn, like steel wool, will burn in an oxygen atmosphere. •Only equipment "cleaned for Oxygen service" should be used for gaseous or liquid oxygen. Never use oil or organic lubricants on oxygen valves and regulators. Pure oxygen reacts about five times faster than air in oxidizing organic materials. Spontaneous combustion is likely to occur. •Care is necessary in housekeeping and product handling so as not to allow oxygen to contact organic materials or any flammable materials. Paints, thinners, and cleaning solvents also must be kept away from oxygen exposures and no ignition sources should be allowed in areas where oxygen is stored or used. No smoking is allowed in areas where oxygen is used and stored. •Use clean gloves or hands washed free of any oils or grease when handling oxygen equipment. Liquid oxygen is very cold and causes cryogenic "burns". The eyes and lungs are especially sensitive to the cold vapors. Protect eyes and skin from exposure to low temperature materials with safety goggles, loose fitting gloves and protective clothing. •Oxygen is nontoxic, but high concentrations may damage the respiratory tract over time. •Clothing which has absorbed liquid oxygen or oxygen gas must be removed and aired out for at least 30 minutes before it is considered safe to wear. Acetylen Characteristics Technical specification Production Safety • Acetylene is a colorless and tasteless gas with a garlic-like odor. It is flammable and can be an asphyxiant. It is one of the fuel gases used in oxy-fuel gas welding, which is any welding procedure that combines a fuel gas with oxygen to produce a flame. The heat and temperature produced by an acetylene flame depend upon the amount of oxygen used to burn it. Air-acetylene produces a flame temperature of around 4000° F (2200° C). This is hot enough to solder aluminum work glass, repair radiators and braze plumbing fixtures. It is not hot enough to weld steel. When acetylene is burned in pure oxygen, the flame temperature may be as high as 5730° F (3166° C). However, the flame temperature and the amount of heat generated (measured as BTUs or kilogramcalories) depend upon the ratio of oxygen to acetylene used. Acetylene can produce carburizing, reducing, neutral and oxidizing flames. The specifications for acetylene are found in the Compressed Gas Association (CGA) Pamphlet G1.1. Grade D (98.0 percent) is considered "commercial" acetylene. The usual grade is about 98.8 percent acetylene. This is the standard acetylene welding grade. Purified acetylene (99.6 percent) is also available. Caution: Never use acetylene at a regulator pressure higher than 15 psig. This fuel gas is sensitive to shock and may explode at higher regulator pressures. Acetylene is not supplied as a liquid for similar safety reasons. The gas is dissolved in acetone and supplied in heavy-walled cylinders filled with porous mass packing material. Purified acetylene (Grade 26) is prepared for use in Atomic Absorption Spectrophotometers. Acetylen Characteristics Technical specification Production Safety Safety Acetylene mixed with air or oxygen in a confined space will explode when ignited. Acetylene decomposes explosively if piped at pressures above 15 psig and exposed to mechanical shock or ignition source. Acetylene forms explosive compounds with copper, silver and mercury. Use steel pipe and fittings and pressure gauges with steel or stainless steel bourdon tubes. Copper alloys if used must contain less than 65 percent copper. Acetylene has a very wide flammability range in air of 2.5 percent to 81 percent by volume. Also very low energy sparks such as static electricity can cause ignition and explosion. Store acetylene cylinder outdoors or in well-ventilated areas away from hot surfaces or flammable materials and ignition sources such as flames or any equipment that can generate a spark. Cylinders must be stored in an upright position. Acetylene cylinders should not be dropped or handled in such a manner as to damage the filter. Use only cylinders and equipment especially designated for acetylene. Never attempt to put acetylene in any other container, equipment or pipeline at pressures above 15 psig. This can be done only at filling plants with proper manifolds, flash arrestors and cylinders with acetone solvent. Make certain all hardware is steel or brass with copper content below 65 percent. Also no silver or mercury can be present where acetylene can react with it. Correct any leak situations. Leaking cylinders that cannot be stopped should be placed outdoors and returned for repair. All electrical equipment must be explosion-proof. Tools used around acetylene must be non-sparking (brass or aluminum bronze is required). Articles of clothing that develop static charges should not be worn where acetylene is handled in volume and leakage may be present. Work in well-ventilated areas. Acetylene is nontoxic but if it displaces oxygen in the air to levels below about 19.5 percent it can cause brain damage and even death. However, acetylene has a distinctive odor which is readily detected in low concentrations so that warning is given of the possible hazard. PHYSICAL PROPERTIES Specific gravity (air = 1) @ 60° F (15.6° C) 0.906 0.554 1.55 1.476 Vapor pressure at 70° F psig (20° C, kPa) 635 (4378) -- 120 (757) 133 (916) Boiling range °F (°C) 1 atm -84 -259 -44 BP -54 BP Flame temperature in O2 °F (°C) 5589 (3087) 4600 (2538) 4579 (2526) 5193 (2867) Latent heat of vaporization at 25° C, Btu/lb, (kJ/kg) -- -- 184 (428) 188 (437) Total heating value (after vaporization) Btu/lb (kJ/kg) 21,500 (50,000) 23,900 (56,000) 21,800 (51,000) 21,100 (49,000) Btu/ft3 (MJ/m3) 1470 (55) 900-1000 (34-37) 2498 (93) 2371 (88) 2404 (90) Safety Acetylene mixed with air or oxygen in a confined space will explode when ignited. Acetylene decomposes explosively if piped at pressures above 15 psig and exposed to mechanical shock or ignition source. Acetylene forms explosive compounds with copper, silver and mercury. Use steel pipe and fittings and pressure gauges with steel or stainless steel bourdon tubes. Copper alloys if used must contain less than 65 percent copper. Acetylene has a very wide flammability range in air of 2.5 percent to 81 percent by volume. Also very low energy sparks such as static electricity can cause ignition and explosion. Store acetylene cylinder outdoors or in well-ventilated areas away from hot surfaces or flammable materials and ignition sources such as flames or any equipment that can generate a spark. Cylinders must be stored in an upright position. Acetylene cylinders should not be dropped or handled in such a manner as to damage the filter. Use only cylinders and equipment especially designated for acetylene. Never attempt to put acetylene in any other container, equipment or pipeline at pressures above 15 psig. This can be done only at filling plants with proper manifolds, flash arrestors and cylinders with acetone solvent. Make certain all hardware is steel or brass with copper content below 65 percent. Also no silver or mercury can be present where acetylene can react with it. Correct any leak situations. Leaking cylinders that cannot be stopped should be placed outdoors and returned for repair. All electrical equipment must be explosion-proof. Tools used around acetylene must be nonsparking (brass or aluminum bronze is required). Articles of clothing that develop static charges should not be worn where acetylene is handled in volume and leakage may be present. Work in well-ventilated areas. Acetylene is nontoxic but if it displaces oxygen in the air to levels below about 19.5 percent it can cause brain damage and even death. However, acetylene has a distinctive odor which is readily detected in low concentrations so that warning is given of the possible hazard.