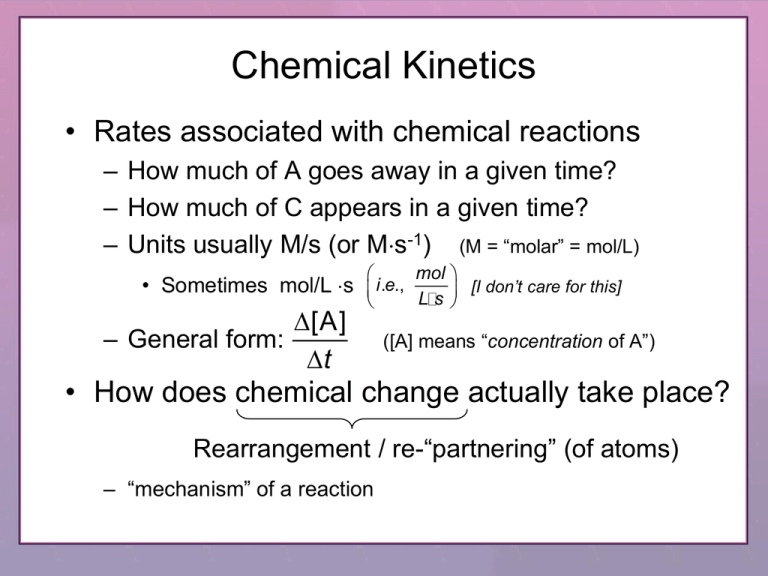
Chemical Kinetics
• Rates associated with chemical reactions
– How much of A goes away in a given time?
– How much of C appears in a given time?
– Units usually M/s (or Ms-1) (M = “molar” = mol/L)
mol
• Sometimes mol/L s i .e.,
L s
[A]
– General form:
t
[I don’t care for this]
([A] means “concentration of A”)
• How does chemical change actually take place?
Rearrangement / re-“partnering” (of atoms)
– “mechanism” of a reaction
Some Early Goals
• Understand concept of reaction rate
• Define various rates
– Of loss, of formation
– Average vs. instantaneous
• Be able to relate “rate of A” to “rate of B (C,
D, etc.)” for a given reaction
– Related by stoichiometry (coefficients)
• Calculate rates of loss or formation from plots
of [ ] vs. time
Copyright © Houghton Mifflin Company. All rights reserved.
12–2
Figure 12.1
(in Zumdahl! Old text;
analogous with Fig. 13.2
in Tro)
Concentration vs.
time plot for a
given reaction.
Can you figure
out the balanced
equation for the
reaction that is
occurring?
Copyright © Houghton Mifflin Company. All rights reserved.
12–3
Balanced Equation?
Pick a given time interval to focus on (see board, ICF)
• Twice as many moles of NO appear as O2
(in that given time interval)
rate of formation of NO is twice that of O2
ratio of those coefficients must be 2 : 1
• The same amount of NO2 is lost as the
amount of NO that is produced (same rates)
coefficients must be same (i.e., 1:1; 2:2)
• Balanced equation is thus:
2 NO2 (g) → 2 NO(g) + O2 (g)
Copyright © Houghton Mifflin Company. All rights reserved.
12–4
Figure 12.2 (Zumdahl) Representation of a
Reaction Represented By:
2 NO2 (g) → 2 NO(g) + O2 (g)
Copyright © Houghton Mifflin Company. All rights reserved.
12–5
Example(s) 1
A + 2B → 3C +D
• If the average rate of decomposition of A is
5.4 x 10-2 M/s over a given time interval, what
is the rate of formation of C over that same
time interval?
• Write an equation showing the relationship
between the rate of formation of C and the
rate of formation of D.
Copyright © Houghton Mifflin Company. All rights reserved.
12–6
Example(s) 2
•
a)
b)
c)
d)
If the rate of formation of B at some time
equals 3.4 M/s and the rate of loss of A
equals 1.7 M/s during that same time
interval, which could be the balanced
chemical equation for the reaction?
A → B
2A→B
A→2B
None of the above
Copyright © Houghton Mifflin Company. All rights reserved.
12–7
Backtrack a bit: specifics on defining
“rates”
From handout:
• Consider a reaction represented by:
aA + bB cC + dD (A, B, C, & D are (aq) or (g))
Negative sign makes the value positive
Copyright © Houghton Mifflin Company. All rights reserved.
12–8
(Also from handout)
Copyright © Houghton Mifflin Company. All rights reserved.
12–9
Figure 12.1
(revisit)
Is the rate of loss
of NO2 constant
as time goes by?
Look at different
time intervals (first
just “eyeball” them; no
calcs yet):
1st 50 s?
3rd 50 s?
5th 50 s?
Copyright © Houghton Mifflin Company. All rights reserved.
12–10
Since rate is constantly changing, must
distinguish “average” rate from
“instantaneous” rate
• The rate of reaction at t = 0 s is not the
same as the rate at t = 50 s!
– The following calculation is an “average”
rate for the interval t = 0 – 50 s:
[NO2 ]
Rate of loss of NO2 =
t
0.0021 M
0.0079 M - 0.0100 M
-5
4.2
x
10
M/s
50. s - 0 s
50. s
Copyright © Houghton Mifflin Company. All rights reserved.
12–11
Table 12.2 Average Rate (in M/s) of
Decomposition of Nitrogen Dioxide as a
Function of Time
Copyright © Houghton Mifflin Company. All rights reserved.
12–12
To calculate an “instantaneous” rate,
draw a tangent line (and find its slope)
• See board (Review of “slope” concept)
• A line is characterized by a single slope
y = mx + b; m is slope or “steepness”
• If y = [A] and x = t, then slope “rate”!
On a plot of [X] vs. t, |slope| = rate
• Curves don’t have a single slope—but:
– each point on a curve can be said to have
a slope equal to the slope of the line
tangent to the curve at that point.
Copyright © Houghton Mifflin Company. All rights reserved.
12–13
See Next Two Slides for illustration
Copyright © Houghton Mifflin Company. All rights reserved.
12–14
Tangent line to
the curve at t =
100 s
Summary: to calculate an “instantaneous
rate” (at a given time) from a plot of [X] vs. t:
1. Draw a tangent line to the curve at the
point associated with the desired time
2. Pick any two points on that tangent line
(better if they are somewhat far apart from one
another)
3. Calculate [X]/t
two points.
•
(slope) using
those
The absolute value of this slope is the
rate of interest (i.e., loss or formation)
Copyright © Houghton Mifflin Company. All rights reserved.
12–17
Figure 12.3 (and Table 12.3)
Copyright © Houghton Mifflin Company. All rights reserved.
12–18
Distinguishing “Reaction Rate” from
Rate of loss or formation of a species
• “Rates” on prior slides were always “rate
of loss” or “rate of formation” of “X”.
– These rates vary for A, B, C, D because of
stoichiometric coefficients
• To get one unambiguous rate of reaction:
– Divide “rate of loss” or “rate of formation” by
coefficient in the balanced equation
– See handout (and email!), and next slide
Copyright © Houghton Mifflin Company. All rights reserved.
12–19
Definition of Rate of Reaction
• For a reaction with equation:
aA + bB cC + dD (A, B, C, & D (aq) or (g))
• The rate of reaction =
Makes rate of change positive
for a reactant (rate of loss)
Rate of change of a product = Rate of formation of it
Divides rate of formation by its coefficient.
Copyright © Houghton Mifflin Company. All rights reserved.
12–20
Example(s) 3
• See Handout Sheet with plot
Copyright © Houghton Mifflin Company. All rights reserved.
12–21
Next Step: What things do you think
should affect the rate of a reaction?
• (on board, first)
• [ ]’s of reactants (Why?
• T (Why? Theory. Details later.
Theory [later])
Takes energy to
break bonds, yes?)
• “Activation Energy”, Ea (later; part of theory)
• Presence of a catalyst (later)
Copyright © Houghton Mifflin Company. All rights reserved.
12–22
(Differential) Rate
Laws
(and how to determine them)
• Definition and symbols (see board)
• How do you find orders and k?
– Find orders using “Method of Initial Rates”
• Do different trials with different initial
concentrations
• See how the initial rate varies
– Use “short method” (if numbers “easy”)
– Use “brute force” method (if necessary [e.g., in lab])
– Find k using substitution (only AFTER the
orders have been determined
Copyright © Houghton Mifflin Company. All rights reserved.
12–23
Practice I with Rate Laws
Write the rate law (format only; no values for
orders and k) for the following:
1) 2 HI(g) H2(g) + I2(g)
Rate Law: R = k[HI]m
2) 2 C2H4(g) + O3(g) 2 CH2O(g) + ½ O2(g)
Rate Law:
R = k[C2H4]m[O3]n
3) 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)
Rate Law:
R = k[NO]m[H2]n
Copyright © Houghton Mifflin Company. All rights reserved.
12–24
Meaning of “orders”
(see boardwork)
The order indicates how sensitive the rate is to
changes in concentration of a given reactant:
R = k[A]
m
1) Zeroth Order—Rate is not dependent on [A]
2) First Order—Rate is proportional to [A]
3) Second Order—Rate is proportional to [A]
squared (i.e., [A]2)
4) Third Order—Rate is proportional to [A] cubed
Copyright © Houghton Mifflin Company. All rights reserved.
12–25
Example Problem on Handout
a)
b)
c)
d)
e)
f)
Doubling the concentration of both A and B
Tripling the concentration of both A and B
Tripling the concentration of A and doubling B
Tripling the concentration of B and doubling A
Halving the concentration of both
Halving the concentration of A and doubling B
Copyright © Houghton Mifflin Company. All rights reserved.
12–26
Initial Rates Data
1) Find the orders of NH4+ and NO22) Calculate k (on board)
NO2-
Copyright © Houghton Mifflin Company. All rights reserved.
12–27
Example Problem 2 on Handout
Determine the rate law (in terms of the rate of
formation of CH2O) and the value of k.
Copyright © Houghton Mifflin Company. All rights reserved.
12–28
Table 12.5 Another Set of Initial Rates Data
Copyright © Houghton Mifflin Company. All rights reserved.
12–29
“Brute force” method
• Calculate
R(trial x)
R(trial y)
(from given data)
• Create an equation by putting the above
on the left side of the equal sign, and
then substituting into the numerator and
denominator on the right side using the
rate law:
R (trial z) = k [A](trial z)m [B](trial z)n
• Do on board (if not yet done)
Copyright © Houghton Mifflin Company. All rights reserved.
12–30
Calculation of k (if not yet done)
R = k[C2H4]m[O3]n
• Earlier found that n = 1 and m = 1 Now find k:
• Pick any trial you want, and substitute values into rate law:
• (Trial 1): 1.0 x 10-12 M/s = k(1.0 x 10-8 M)1(0.50 x 10-7 M)1
1.0 x 10-12 M/s
3
-1 -1
k
k
2.0
x
10
M
s
-8
-7
(1.0 x 10 M)(0.50 x 10 M)
Careful with units!
Copyright © Houghton Mifflin Company. All rights reserved.
12–31
Calculation of k (if not yet done)
• Remember, k has units!
– The units of k are not “fundamental”—they
change depending on the overall order of
the reaction of interest. Use algebra:
1st order overall: R = k[A] k = R/[A]
2nd order overall: M-1s-1
3rd order overall: M-2s-1
Etc.
Copyright © Houghton Mifflin Company. All rights reserved.
M
s
M
1
s 1
s
12–32
Practice / Review of logs and exponential functions
(Problems will be done on board in class)
•
•
•
•
logba = x bx = a; b is “base”; a is “argument”
If no “b” present, it is an implied 10 (i.e., base 10 log)
loge = ln (i.e., “natural log”); e is a special number (like p)
lnx is the inverse of ex; “undo” one another: ln(ex) = x !
Copyright © Houghton Mifflin Company. All rights reserved.
12–33
Integrated Rate Laws
(How does [ ] vary with time?)
• If -[A]/t (rate of loss of A) depends on [A],
then [A] must also depend on t
– Precise relationships (for each situation: 0th, 1st,
and 2nd order) are given by calculus (which is not
required for this course)
– However, much can be conceptually rationalized
without calculus, and that will be my focus.
– At least one equation will need to be memorized; I
will stress/show how to derive others from it.
• Board Work
(0th , 1st , and concept of “half life”)
Copyright © Houghton Mifflin Company. All rights reserved.
12–34
• A first order reaction is 35% complete at
the end of 55 minutes. What is the value
of k?
Copyright © Houghton Mifflin Company. All rights reserved.
12–35
Half Life “Quick Quiz”
What is the half life for this reaction (trial)?
0.9
Answer: About 2.5 s
0.8
0.7
[A] (M)
0.6
0.5
0.4
0.3
0.2
0.1
0
0
5
10
15
20
25
30
35
Time (s)
Copyright © Houghton Mifflin Company. All rights reserved.
12–36
Figure 12.7
A Plot of [A]
versus t for a
Zero-Order
Reaction
[A]
R k [A]0 k
t
Note: k is always
a positive qty.
The negative sign
makes it + (b/c
slope is negative)
Copyright © Houghton Mifflin Company. All rights reserved.
12–37
[ ] vs. t Plot for
2 N2O5 → 4 NO2 + O2
0.12
[N2O5] (M)
0.1
0.08
0.06
0.04
0.02
0
0
100
200
300
400
500
Time (s)
Is the reaction 0th order in N2O5? 1st order?
How do you know? (See next slide as well)
Copyright © Houghton Mifflin Company. All rights reserved.
12–38
Figure 12.4 A Plot of In[N2O5] versus
Time
Copyright © Houghton Mifflin Company. All rights reserved.
12–39
Table 12.6 Summary of Kinetics Info on
0th, 1st, and 2nd Order Reactions
(A bit later)
Copyright © Houghton Mifflin Company. All rights reserved.
12–40
[A]t
e kt
[A]0
Fig. 13.11, Tro
[A]t [A]0 e kt
2nd Order Integrated Rate Law
• Back to board
• Use idea that in 2nd order:
“rate is more sensitive to changes in [A] than
in 0th or 1st order cases”
Gets “increasingly” slower as time goes by!
Not “exponential” decay; decays “more
slowly”
Half life gets longer and longer as [A] gets
smaller and smaller (takes “forever” to get
to zero—see next slide)
Copyright © Houghton Mifflin Company. All rights reserved.
12–43
2nd Order
What is the second half life for this reaction (trial)?
0.9
The 3rd half life?
0.8
0.7
For 2nd order processes, half life
increases with time (really,
increases as [A]o decreases)
[A] (M)
0.6
0.5
0.4
0.3
0.2
0.1
t1 / 2 ( 3)
t1/ 2 (1) t1/ 2 ( 2)
0
0
5
10
15
20
25
30
35
Time (s)
Copyright © Houghton Mifflin Company. All rights reserved.
12–44
Fig. 13.5, Tro
Figure 12.6 (a) A Plot of In[C4H6] versus t
(b) A Plot of 1/[C4H6] versus T
Copyright © Houghton Mifflin Company. All rights reserved.
12–46
[A]t
e kt
[A]0
Copyright © Houghton Mifflin Company. All rights reserved.
12–47
Mechanisms
• See Handout (in part)
Copyright © Houghton Mifflin Company. All rights reserved.
12–48
Mechanism for the Iodination of Acetone (Exp 20)
+
O
O
Step 1
+ H3 O +
CH3CCH3
k1
H
+
(fast, equilibrium)
CH3CCH3
H
+
O
H
H
O
Step 2
CH3C
H +
C
H
k2
H2O
CH3C
C
H
H
O
O
H
CH3C
+
C
I
I
H
k3
CH3C
C
H
Step 4
CH3C
O
H
C
H
+ H2O
H
+
I-
(fast)
I
H
O
(slow)
H3O
H
H
Step 3
+
+
k4
I
Copyright © Houghton Mifflin Company. All rights reserved.
CH3C
H
C
H +
+
H3O
(fast)
I
12–49
Mechanism Ideas Discussed Earlier
• For elementary reactions (steps) only, the
rate law is “knowable” from the balanced
equation of the elementary step.
• The rate of an overall reaction that occurs in
more than one step can only be as fast as the
slowest step:
Roverall Rslow
(Rslow is also called Rrls or Rrds)
Copyright © Houghton Mifflin Company. All rights reserved.
12–50
Mechanism Ideas Discussed Earlier (Cont’d)
• A mechanism dictates (predicts) an overall
rate law for the reaction
• If the predicted rate law does NOT match the
experimental rate law (the “actual” rate law), the
proposed mechanism is “wrong” (rxn does not occur
by that mechanism)
• If the predicted rate law DOES match
experiment, the mechanism is “possibly”
correct, but not necessarily correct.
– More than one mech can predict the same rate law!
Copyright © Houghton Mifflin Company. All rights reserved.
12–51
The Rate Law for an Elementary Step Has
Orders Equal to Coefficients
Elementary occurs in one collision
Every collision “matters”
[recall the iodination reaction—collisions after the slow
step did not “matter” because the rate was limited by
the slow step]
Twice as many collisions means twice the
reaction rate. 2x the [ ] means 2x the collisions!
Order is 1 for each species involved in the
collision.
NOTE: If a species is involved twice in the
collision [i.e., it collides with itself], its order will
be 2.
Copyright © Houghton Mifflin Company. All rights reserved.
12–52
Table 13.3 (Tro) Examples of
Elementary Steps and Their Rate Laws
NOTE: You can “know” the rate laws for elementary steps only
(using collision theory—higher [ ] more collisions)
Copyright © Houghton Mifflin Company. All rights reserved.
12–53
Examples (from Handout)
• What are the rate laws predicted by:
– Mechanism 1?
R = k[NO2][F2] (Rslow)
– Mechanism 2?
R = k[NO2]2[F2] (Rslow)
– Mechanism 3?
R = k[NO2][F2] (Rslow)
If the actual rate law is R = k[NO2][F2],
what can you conclude? #2 is not the mechanism!
Copyright © Houghton Mifflin Company. All rights reserved.
12–54
Temperature Dependence of k—Ea and
the Arrhenius Equation
• Board Work (PE curves, etc.)
Copyright © Houghton Mifflin Company. All rights reserved.
12–55
Figure 12.10 (Zumdahl)
A Plot Showing
the Exponential
Dependence of
the Rate
Constant on
Absolute
Temperature
Arrhenius
Equation (“Law”):
k Ae
Ea
RT
A is the “Arrhenius constant” or
“frequency factor”
Copyright © Houghton Mifflin Company. All rights reserved.
12–56
Figure 12.11 a & b (Zum.)
(a) The Change in Potential Energy as a Function of
Reaction Progress
(b) A Molecular Representation of the Reaction
Copyright © Houghton Mifflin Company. All rights reserved.
12–57
How can one determine Ea
experimentally?
• Take ln of both sides of the Arrhenius
equation
• Swap lnA term with the other term on right
• Get the following (see board [and posted file]):
Ea 1
ln k
ln A
R T
y
m x
b
Plot lnk vs. 1/T ; Find slope, set equal to –Ea/R !
12–59
Example 13.7 in Tro
ln k (no units)
1/T (K-1)
8.123
0.001667
10.79
0.001429
12.79
0.00125
14.35
0.001111
15.59
0.001000
16.61
0.000909
17.46
0.000833
18.18
0.000769
18.79
0.000714
19.32
0.000667
19.79
0.000625
20.20
0.000588
20.57
0.000556
20.90
0.000526
12–60
m
Ea
Ea mR (1.12 x 10 4 K )(8.314 J K -1 mol -1 )
R
93116.8 J/mol 93.1kJ/mol
ln k
1/T (K-1)
8.123
0.001667
10.79
0.001429
12.79
0.00125
14.35
0.001111
15.59
0.001000
16.61
0.000909
17.46
0.000833
18.18
0.000769
18.79
0.000714
19.32
0.000667
19.79
0.000625
20.20
0.000588
20.57
0.000556
20.90
0.000526
12–61
Collision Theory Explains
Arrhenius Equation Behavior
• In order for a collision to lead to reaction
(products being formed):
– It must have enough energy
– It must have the correct orientation
Copyright © Houghton Mifflin Company. All rights reserved.
12–62
Collision Theory Explains
Arrhenius Equation Behavior
k Ae
Ea
RT
• Exponential factor: Fraction of collisions
with enough energy to react
• Frequency Factor: Number of times per
second a collision with the correct orientation
occurs [when reactants are at 1 M] (sort of; Tro calls this the
“number of approaches to the transition state per sec”; a bit unclear).
Copyright © Houghton Mifflin Company. All rights reserved.
12–63
Collision Theory Explains
Arrhenius Equation Behavior
k Ae
Ea
RT
pze
Ea
RT
• Exponential factor: tells fraction of collisions that
have a KE great enough for reaction to occur (≥ Ea )
Value goes from 0 – 1; depends on T!
Greater T, greater exponential factor (b/c avg KE
T greater T, greater KE
• Steric (orientation) factor (p): tells fraction of
collisions that have the proper orientation to make
products
Value typically goes from 0 – 1 (text notes exception)
Copyright © Houghton Mifflin Company. All rights reserved.
12–64
Graphical / Physical Interpretation of the
Exponential Factor
• (see next slide)
Copyright © Houghton Mifflin Company. All rights reserved.
12–65
Figure 13.14 (Tro): Plot Showing the
Fraction of Collisions with a Particular
Energy at T1 and T2, where T2>T1
The fraction spoken about
here (represented by the colored
areas under the curve) is equal
to the value of the
exponential factor:
Number
e
Ea
RT
whose value goes from:
0 (at T = 0) to 1 (as T )
Kinetic
Copyright © Houghton Mifflin Company. All rights reserved.
*NOTE: This fraction depends
on two things: Ea and T.
12–66
# of particles (with a given KE)
Actual shapes of KE distribution curves for
a sample at two temperatures
Note that the areas under the
curve are the same, but the
peak for the lower T curve is at
a much smaller KE
0
5000
10000
15000
20000
25000
KE (arbitrary units)
Copyright © Houghton Mifflin Company. All rights reserved.
12–67
KEcollision ≥ Ea
Average KE
increases with T,
so more collisions
have a higher KE.
An intrinsic property of
the reaction (mechanism)
[does not change with T]
KEcollision < Ea
Graphical / Physical Interpretation of the
Steric (Orientation) Factor (p)
• (see board, then next slide)
Copyright © Houghton Mifflin Company. All rights reserved.
12–69
What kinds of orientations at the point
of collision would lead to reaction?
Hint: Which bonds are made and broken?
p probably < 0.2
What is a catalyst and how does it “work”?
• Typical definition: A species that speeds up a
reaction without being consumed.
– Okay, but really only part of the story
• how does it speed up the reaction?
• How can it not be consumed? (Some even say it is “not
involved in the reaction” How silly! Is it “magic”?!)
Copyright © Houghton Mifflin Company. All rights reserved.
12–72
Catalysts (cont.)
• Some say that a catalyst “lowers the
activation energy” for a reaction.
– That’s roughly true, but not precisely true.
– It cannot change the activation energy of “the
exact process”, because the activation energy is
determined by the collisions in that process…
• A catalyst changes the mechanism of a
reaction! Creates a new pathway that
avoids the original reaction’s “slow step”!
– The new pathway’s activation energy is generally smaller
than the original one’s. So there is truth to the idea noted
above. But see next slide for an oversimplification…
Copyright © Houghton Mifflin Company. All rights reserved.
12–73
A catalyst creates a new
mechanism (pathway) that has a lower overall Ea
Figure 12.15 (Zumdahl):
In actuality,
the catalyzed
pathway must
have at least
one intermediate (it
can’t be one
step!)
Copyright © Houghton Mifflin Company. All rights reserved.
12–74
Addition of a Catalyst
Increases the Number of Collisions That
Have the Energy Needed to React (i.e., to
Figure 12.16 (Zumdahl):
“Get Over” the Activation Energy Barrier) without
raising the temperature
Copyright © Houghton Mifflin Company. All rights reserved.
12–76
Ozone “Hole” Forms over Antarctica
because of Cl in atmosphere getting trapped
in “Polar Vortex”
May, 2004
October, 2004
An Enzyme is Biological Catalyst!

