Chapter 17 - WalliDhama.com
advertisement

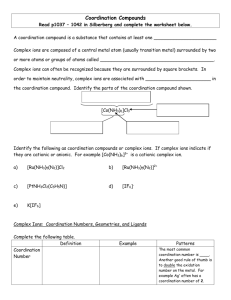
Chapter 17 The Colorful Chemistry of Transition Metals Lewis Acids and Bases • A Lewis base is a substance that donates a pair of electrons in a chemical reaction. • A Lewis acid is a substance that accepts a pair of electrons in a chemical reaction. Figure 17.1 Another Lewis Example Complex Ions • A complex ion is an ionic species consisting of a metal ion bonded to one or more Lewis bases. • A coordinate bond forms when one anion or molecule donates a pair of electrons to another ion or molecule to form a covalent bond. • A ligand is a Lewis base bonded to the central metal ion of a complex ion. • The inner coordination sphere of a metal consists of the ligands that are bound directly to the metal via coordinate covalent bonds. Complex Ions Dissolved metal ions are Lewis Acids, and form aqueous complexes with Lewis Bases More Metal Complexes Terms • Counter ions are the ions that balance the electrical charges of complex ions in coordination compounds (e.g. in basic solution OH- ions could act as counter ions Zn(NH3)4(OH)2 • Coordination compounds are made up of one or more complex ions; typically no more than 6 ligands surround the metal. • The coordination number (CN) of a metal ion identifies the number of electron pairs surrounding it in a complex. Which ions are the counter ions in the following: Na2[Zn(CN)4] [Co(NH3)4Cl2]NO3 Bonding in Zn(NH3)42+ Ion Common Coordination Numbers and Shapes for Complex Ions Coordinationnu mber Shape Hybridization Example 6 Octahedral d2sp3 Fe(H2O)63+ 4 Tetrahedral sp3 Zn(H2O)42+ 4 Square Planar dsp2 Pt(NH3)42+ 2 Linear sp Ag(NH3)2+ Are any of these NOT Lewis bases? Would you expect H2O or NH3 to be the stronger Lewis Base? Complex-Ion Equilibria Cu2+(aq) + 4 NH3(aq) <==> Cu(NH3)42+(aq) Figure 17.5 2 + Cu(NH ) 3 4 13 Kf = = 5.0 x 10 4 2 + Cu NH 3 17.26. When a strong base is added to a solution of CuSO4, which is pale blue, a precipitate forms and the solution above the precipitate is colorless. When ammonia is added, the precipitate dissolves and the solution turns a deep navy blue. Use appropriate chemical equations to explain why the observed changes occurred. Formation Reactions The equilibrium constants associated with complexation are called formation constants Kf = [Cu(NH3)42+]/[Cu2+][NH3]4 = 5.0E+13 Metal ions as Lewis Acid also promote hydrolysis of water, and the formation of H3O+ Metal cations (Fe3+, Cr3+ and Al3+)with large positive charges are more likely to cause hydrolysis. Ka Values of Hydrated Metal Ions Ion Ka Fe3+(aq) 3 x 10-3 Cr3+(aq) 1 x 10-4 Al3+(aq) 1 x 10-5 Cu2+(aq) 3 x 10-8 Pb2+(aq) 3 x 10-8 Zn2+(aq) 1 x 10-9 Co2+(aq) 2 x 10-10 Ni2+(aq) 1 x 10-10 17.45. Sketch the titration curve (pH versus volume of 0.50 M NaOH) for a 25 mL sample of 0.5 M FeCl3 (Ka=3E-3) 17.5. Solubility of Ionic Compounds Minerals in contact with ground water will dissolve to some extent. CaCO3(s) ↔ Ca2+(aq) + CO32-(aq) Write the equilibrium expression for this dissolution. Solubility Equilibria CaCO3(s) ↔ Ca2+(aq) + CO32-(aq) K = [Ca2+(aq)][CO32-(aq)] = Ksp The equilibrium expression is called the solubility product (sp), because it involves only products of the concentrations of the dissolved species and NOT the solid. If Ksp is known, the solubility (at equilibrium) of the solid can be calculated Solubilities of Ionic Compounds The solubility-product constant (Ksp) is an equilibrium constant that describes the formation of a saturated solution of a slightly soluble salt. Mg(OH)2(s) <==> Mg2+(aq) + 2 OH-(aq) Ksp = [Mg2+][OH-]2 = 5.6 x 10-12 Ksp values of some common salts HgS(s) Fe(OH)3(s) AgI(s) Ksp = [Hg2+][S2-] = 4.0 x 10-53 Ksp = [Fe3+][OH-]3 = 2.8 x 10-39 Ksp = [Ag1+][I1-] = 8.5 x 10-17 CaCO3(s) CaSO4(s) Ag2SO3(s) Ksp = [Ca2+][CO32-] = 9.8 x 10-9 Ksp = [Ca2+][SO42-] = 4.9 x 10-5 Ksp = [Ag1+]2[SO32-] = 1.2 x 10-5 NaCl(s) Ksp = [Na1+][Cl1-] = 6.2 Solubility Problem 17.58. What are the equilibrium concentrations of Pb2+ and F– in a saturated solution of lead fluoride if the Ksp value of PbF2 is 3.2 × 10–8? Solubility Problem 2.75 grams of BaF2 (FW=175.3) is placed in enough water to make 1.00 L at 25°C. After equilibrium has been established…the F- concentration equal 0.0150 M, what is the Ksp for BaF2. Solubility Problem 50 mg of PbSO4 (FW=303.3) is placed in 250 mL of pure water; What percentage of the solid dissolves? Ksp(PbSO4)=1.8E-8 (Table A5.4) Solubility Problem Calculate the pH of a saturated solution of zinc hydroxide, Ksp = 4.0E-17 Solubility Problem 120 Calculate the solubility of silver chloride in seawater with a chloride concentration of 0.547 M. Ksp(AgCl) = 1.8E-10 Terms • A monodentate ligand is a species that forms only a single coordinate bond to a metal ion in a complex. • A polydentate ligand is a species that can form more than one coordinate bond per molecule. • Chelation is the interaction of a metal with a polydentate ligand (chelating agent). (e.g. Google “chelation therapy”) Chelation-Examples (a) bidentate chelation Ni2+(aq) by ethylenediamine (b) 3 ethylenediamime molecules bind to Ni2+ tridentate chelation by diethylenetriamine. A Hexadentate Ligand Ethylenediaminetetraacetic acid (EDTA) forms 6 stable, coordinate bonds with many metals Ligands and Complex Colors NiCl2 [Ni(NH3)]Cl2 Complex Ions of Nickel • Ni(H2O)62+ is equivalent to Ni2+(aq) and is green in solution. Ni2+(aq) + 6NH3(aq) <==> Ni(NH3)62+(aq) Kf = 5 x 108 Ni2+(aq) + 3en <==> Ni(en)32+(aq) Kf = 1.1 x 1018 en=ethylenediamine • The chelate effect is the greater affinity of metal ions for polydentate ligands compared to the corresponding monodentate ligands. Crystal Field Theory… why transition metal complexes are colored • Crystal field splitting is the separation of a set of d-orbitals into subsets with different energies as a result of interactions between electrons in those orbitals and pairs of electrons in ligands surrounding the orbitals. • Crystal field splitting energy () is the difference in energy between subsets of d-orbitals split by interactions in a crystal field. d-orbitals in an octahedral field Light (photons) Can Promote Electrons in a Complex Ion’s Orbitals Fig. 17.11: Crystal field spitting of dorbital energies that results from the interaction with p-orbitals in an octahedral geometry. The difference in energy is called the crystal field splitting energy (). An example of this is the substitution of Cr3+ for Al3+ in the octahedral holes of the silicate structure of beryl. The Be2+ sits in tetrahedral holes. Be3CrxAl2-xSi6O18 Review: See chapter 7 for shapes of atomic orbitals. The Color of Compounds • Our eyes perceive the transmitted colors of complex ions. • [Cu(NH3)4]2+ absorbs much of the yellow, orange, and red wavelengths of light, so we see the complex as being navy blue. Color Wheel Ni(H2O)62+ --> Ni(NH3)62+ --> Ni(en)32+ Green Blue Violet • Ni(H2O)62+ is green because it absorbs the color red on the opposite side of the color wheel. • Ni(NH3)62+ is blue because it absorbs the color opposite blue, which is orange. • Ni(en)32+ is violet because it absorbs colors centered on the complement of violet, which is yellow (en is the ethlyenediamine ligand). Crystal Field Splitting Energy must change for the three nickel compounds. • H2O as a ligand must yield the smallest because the red light, which is lowest in energy, is absorbed by the nickel complex. • NH3 must cause a little larger than water because a higher energy of orange light is absorbed. • The ligand en must cause the largest , because its complex absorbs yellow light, which is highest in energy of the three examined. The Spectrochemical Series A list of ligands ordered by their abilities to increase , the crystal field splitting energy of the d-orbitals. Square Planar Crystal Field Splitting How many delectrons does Cu2+ have? Fig. 17.15: The mineral turquoise, CuAl6(PO4)4(OH)84H2O, has Cu2+ ions occupying irregular shaped octahedral holes. Consequently, the crystal field spitting produces four energy levels among the d-orbitals. Tetrahedral Crystal Field Splitting Magnetism and Spin States Magnetism and Spin States Both the high- and lowspin species of Fe(III) complexes are paramagnetic. High-spin species would have a greater interaction with a magnetic field. High-spin complexes result from weak field ligands (H2O); low-spin complexes result from strong-field ligands (CN-) The Spectrochemical Series A list of ligands ordered by their abilities to increase , the crystal field splitting energy of the d-orbitals. Electron Paramagnetic Resonance spectroscopy is a technique for measuring chemical species with one or more unpaired elections. An unpaired electron can move between the two energy levels by either absorbing or emitting electromagnetic radiation of energy ε = hν ΔE = geμBB0 (i.e. the splitting is proportional to the magnetic field strength (B0) Naming Positively Charged Complex Ions • Start with the name of ligand(s) (see Table 17.3 for common ligand names). • Use the usual prefixes to indicate the number of each type of ligand (di(2), tri(3), etc.). • Next write the name of the central metal ion using a Roman numeral to indicate the oxidation state of the transition metal ion. • Ligands are named alphabetically (excluding prefixes). Examples Ni(H2O)62+ Hexaaquanickel(II) Co(NH3)63+ Hexaamminecobalt(III) [Cu(NH3)4(H2O)2]2+ Tetraamminediaquacopper(II) Naming Negatively Charged Complex Ions • Follow the steps for a positively charged complex ion. • Use an -ate ending on the name of the central metal ion to indicate the charge of the complex ion. Examples • Fe(CN)63- Hexacyanoferrate(III) • [Fe(H2O)(CN)5]3Aquapentacyanoferrate(II) • [Al(H2O)2(OH)4]Diaquotetrahydroxoaluminate Coordination Compounds • [Ni(H2O)6]Cl2 Hexaaquanickel(II) Chloride • K3[Fe(CN)6] Potassium Hexacyanoferrate(III) Geometric Isomers cis-Pt(NH3)2Cl2 trans-Pt(NH3)2Cl2 cis-platinum is a widely in chemotherapy to treat cancer, while the trans-isomer is ineffective. Geometric Isomers of octahedral complexes Enantiomers • Enantiomers are geometric isomers that are nonsuperimposable mirror images of each other. • Enantiomeric ions and molecules are called chiral. Metal Complexes in Biomolecules Heme Cytochrome c Protein Metalloporphyrins ChemTour: Crystal Field Splitting Click to launch animation PC | Mac This animation illustrates how transition metals can fill tetrahedral and octahedral holes in crystal lattices, resulting in the splitting of the energies of the d orbitals and the brilliant color of gemstones. • Note to instructors: The following in-class review questions are also applicable to chapter 18, Electrochemistry and Electric Vehicles. • Per the note in chapter 10, Forces between Ions and Molecules and Colligative Properties, the review question posted there is also applicable to this chapter. Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? A) Ni(s) B) Al3+(aq) Oxidizing Strength of Al +, Ni, and Cu + C) Cu2+(aq) Consider the following arguments for each answer and vote again: A. Ni(s), a metallic conductor, can accept and release electrons most readily. B. Al3+(aq) is the strongest oxidizing agent because it has the greatest positive charge. C. Cu2+(aq) can oxidize Ni(s) and Ni2+(aq) can oxidize Al(s), so Cu2+(aq) can oxidize Al(s). Oxidizing Strength of Al +, Ni, and Cu + For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? A) Yes Ag/Cu Electrochemical Cell B) No C) Depends Consider the following arguments for each answer and vote again: A. Under standard conditions, Ag+ can oxidize Cu to form Cu2+. Therefore, electrons will flow from the Cu electrode to the Ag electrode. B. ΔG° is positive, and so the reaction is not spontaneous and no electrons will flow between the two electrodes. C. Whether a current will flow in the electrochemical cell depends on which metal, Cu or Ag, is the anode and which is the cathode. Ag/Cu Electrochemical Cell For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (x-axis)? A) Oxidation of Fe + by Ag+ B) C) Consider the following arguments for each answer and vote again: A. At lower temperatures, the negative enthalpy dominates, so the reaction is more spontaneous. B. All electrochemical reactions have an increased E° at higher temperatures due to the increase in kinetic energy provided to the transferred electrons. C. E° depends only on the concentrations of the species in solution and is independent of the temperature. Oxidation of Fe + by Ag+ For the cell pictured to the left, the voltage, E, is measured to be 0.79 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red = 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 Consider the following arguments for each answer and vote again: A. A lower H3O+ concentration in the right half of the cell would result in a decrease in the efficiency of the cell, causing the cell voltage to drop. B. This electrochemical reaction is spontaneous only under standard conditions, where the concentration of H3O+ is 1.0 M and the pH is 0. C. A pH less than zero favors the formation of H2(g) and thus would decrease the overall voltage of the cell. pH of an Electrochemical Cell For the electrochemical cell Cu | Cu2+(1.0 M) || Cu2+(0.1 M) | Cu, what will happen to the color in the darker solution (1.0 M Cu2+) once the circuit is completed? A) It gets darker. B) It gets lighter. C) It stays the same. Cu/Cu + Concentration Cell Consider the following arguments for each answer and vote again: A. Formation of Cu2+(aq) through the oxidation of Cu occurs in both cells, so both solutions should get darker. B. During the approach to equilibrium, which reduces the cell potential, Cu will plate onto the electrode in the darker solution. C. Both half-cells contain the same type of ions and the electrons do not know in which direction to flow. Cu/Cu + Concentration Cell