Physics 102 Introduction to Physics
advertisement

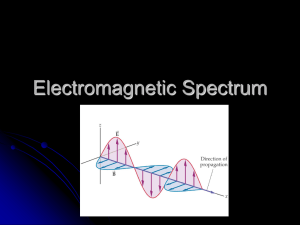
Physics 102-002 Announcements • WebAssign – – Chapter 26 due next Monday • No class Wednesday – We’ll cover Ch 28 next week • Exam 3 Corrections due Wed, Apr 25 Picture: National Science Foundation's Very Large Array (VLA) radio telescope image of a "spectacular and complex structure" in giant elliptical galaxy M87, the central galaxy of the Virgo Cluster of Galaxies. This photo shows two large, bubble-like lobes, more than 200,000 light-years across, that emit radio waves. Class Schedule 4/9 Midterm Exam #3 4/11 Chapter 24 Magnetism, (Pg 458-470) 4/16 Chapter 26 Properties of Light 4/18 No class 4/23 Chapter 28 Reflection and Refraction, Part 1 (Pg 530-540) 4/25 Chapter 28 Reflection and Refraction, Part 2 (Pg 540-551) 4/30 Chapter 30 Light Emission (Pg 558-568) Midterm Exam #4 5/2 Review 5/7 Final Exam Note the changes!!! Chapter 26 Properties of Light • Electromagnetic Waves – Electromagnetic Wave Velocity – Electromagnetic Spectrum • Transparent Materials • Opaque Materials What is Light? When you look at something … anything … you are, in fact, seeing light -- light that somehow left the object far or near and reached your eyes. Light is all our eyes can really see. You also encounter light in devices that produce light -- incandescent bulbs, fluorescent bulbs, lasers, lightning bugs, the sun. Each one uses a different technique to generate photons (or “pieces” of light) Modern physicists believe that light can behave as both a particle and a wave. We will talk about light mostly as waves, because this provides the best explanation for most of the phenomena our eyes can see. There are many different ways to produce photons, but all of them use the same mechanism inside an atom to do it. This mechanism involves the energizing of electrons orbiting an atom's nucleus Electrons circle the nucleus in fixed orbits. An electron has a natural orbit that it occupies, but if you energize an atom you can move its electrons to higher orbitals. A photon of light is produced whenever an electron in a higher-than-normal orbit falls back to its normal orbit. During the fall from high-energy to normal-energy, the electron emits a photon -- a packet of energy -- with very specific characteristics. The photon has a frequency, or color, that exactly matches the distance the electron falls. Electromagnetic Waves If you shake a charged stick in the air, you create a current … a moving charge. Recall that a moving charge emits a magnetic field. A changing magnetic field creates a changing electric field. If the charge moves back and forth (oscillates), it emits an oscillating magnetic field. The oscillating magnetic field creates an oscillating electric field, which creates an oscillating magnetic field, and so on … Both of these fields continuously regenerate each other and propagate out through all space. This is an Electromagnetic Wave, and you can visualize it as shown. The electric and magnetic fields oscillate in planes perpendicular to each other as the wave moves through space, both moving in the same direction at the same speed, and at the same frequency. Physics Place figure Electromagnetic Wave Velocity Light always moves through a vacuum at 3x108 m/s … ALWAYS! (it travels a bit slower when moving through other materials, though) In the 1800s, JC Maxwell was able to use the equations of electrostatics and magnetism to DERIVE the exact value for the speed of light in vacuum WITHOUT measuring it. His calculated value has since been experimentally verified many times over. All electromagnetic radiation moves at this speed! Maxwell discovered that visible light is electromagnetic radiation in the frequency range between 4.3X1014 Hz and 7x1014 Hz (vibrations per second). Other types of electromagnetic radiation (radio waves, x-rays, tv signals, radar, etc) all have the same cause and nature as visible light, but just vibrate at different frequencies. Notice that electromagnetic radiation doesn’t need a medium to move through, it can move through a vacuum! That’s because both electric and magnetic fields are transmitted through space without needing a medium. James C. Maxwell The Electromagnetic Spectrum The classification of electromagnetic waves according to frequency is the electromagnetic spectrum. The EM Spectrum by frequency The EM Spectrum by wavelength Visible light is less than 1 millionth of 1% of the EM spectrum The divisions between regions aren’t sharp, they’re a little fuzzy, and they overlap. Also, some of the regions are further divided. For example, there are Near-, mid-, and far-infrared, and hard and soft x-rays. Frequency and wavelength are related. The higher the frequency, the shorter the wavelength. Further, frequency and wavelength are related to the speed of light. Physics Place figure Frequency x wavelength = speed of light c=3x108 m/s in vacuum Question 1 Question 1 Answer Transparent Materials Recall that light originates from the vibrations of electrons in atoms. When light strikes a material, some of the electrons in the matter are forced to start vibrating at the same frequency as the light that struck the matter. These electrons emit light of the same frequency which strikes adjoining atoms, etc. In this way the light is transmitted through the material. As the electrons in glass are set in vibration by incoming light, they re-emit light at the same frequency Light at a given frequency is absorbed at “preferred” frequencies by atoms in much the same way that sound of a given frequency can start a matching tuning fork vibrating. Because it takes time for atoms to absorb and re-emit light, it takes longer for light to travel through matter than for it to travel through a vacuum. The speed of light in “dense media” depends on the density of the medium. In glass, light travels at 0.67c Light travels at different speeds through oil and water, which is why an oil film on top of water has a “rainbow” effect. Opaque Materials Sometimes light at the right frequency matches the “resonant” frequency of the atoms in matter. In cases like this, the light sets the entire atom or molecule in vibration. When this happens, the electrons don’t undergo a change in energy and light is not re-emitted. Much of the lights energy is lost in heating up the matter. Light doesn’t get transmitted through the matter, and we say the substance is “opaque”. Glass is opaque to infrared and ultraviolet light, but is transparent to visible light. Clouds are transparent to most ultraviolet light, but not visible light, which is why you can get sunburned on a cloudy day. Shadows Distinct shadow (Umbra) caused by small nearby light source or a large far away light source Fuzzy, blurry shadow (Penumbra) caused by small nearby light source or a large far away light source Question 2 Question 2 Answer