B. - LiveText
advertisement
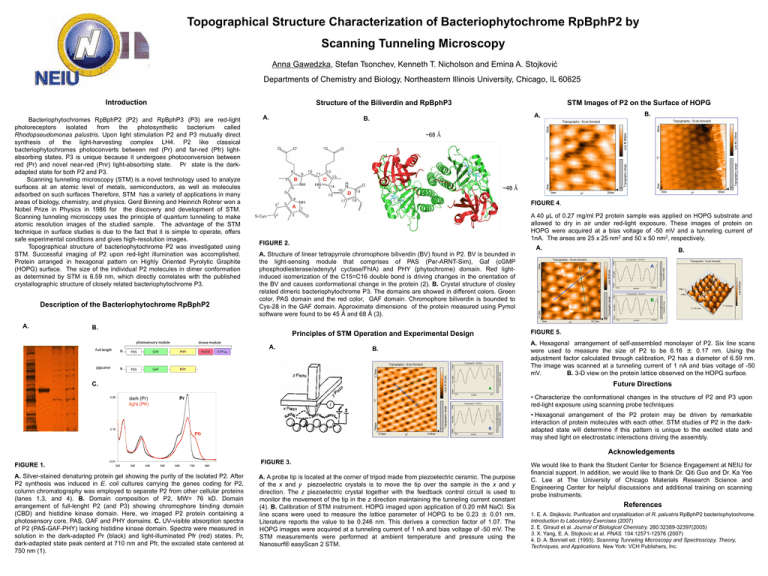
Topographical Structure Characterization of Bacteriophytochrome RpBphP2 by Scanning Tunneling Microscopy Anna Gawedzka, Stefan Tsonchev, Kenneth T. Nicholson and Emina A. Stojković Departments of Chemistry and Biology, Northeastern Illinois University, Chicago, IL 60625 Introduction Structure of the Biliverdin and RpBphP3 Bacteriophytochromes RpBphP2 (P2) and RpBphP3 (P3) are red-light photoreceptors isolated from the photosynthetic bacterium called Rhodopseudomonas palustris. Upon light stimulation P2 and P3 mutually direct synthesis of the light-harvesting complex LH4. P2 like classical bacteriophytochromes photoconverts between red (Pr) and far-red (Pfr) lightabsorbing states. P3 is unique because it undergoes photoconversion between red (Pr) and novel near-red (Pnr) light-absorbing state. Pr state is the darkadapted state for both P2 and P3. Scanning tunneling microscopy (STM) is a novel technology used to analyze surfaces at an atomic level of metals, semiconductors, as well as molecules adsorbed on such surfaces Therefore, STM has a variety of applications in many areas of biology, chemistry, and physics. Gerd Binning and Heinrich Rohrer won a Nobel Prize in Physics in 1986 for the discovery and development of STM. Scanning tunneling microscopy uses the principle of quantum tunneling to make atomic resolution images of the studied sample. The advantage of the STM technique in surface studies is due to the fact that it is simple to operate, offers safe experimental conditions and gives high-resolution images. Topographical structure of bacteriophytochrome P2 was investigated using STM. Successful imaging of P2 upon red-light illumination was accomplished. Protein arranged in hexagonal pattern on Highly Oriented Pyrolytic Graphite (HOPG) surface. The size of the individual P2 molecules in dimer conformation as determined by STM is 6.59 nm, which directly correlates with the published crystallographic structure of closely related bacteriophytochrome P3. Description of the Bacteriophytochrome RpBphP2 A. A. STM Images of P2 on the Surface of HOPG B. ~68 Ǻ ~48 Ǻ FIGURE 4. A 40 μL of 0.27 mg/ml P2 protein sample was applied on HOPG substrate and allowed to dry in air under red-light exposure. These images of protein on HOPG were acquired at a bias voltage of -50 mV and a tunneling current of 1nA. The areas are 25 x 25 nm2 and 50 x 50 nm2, respectively. FIGURE 2. A. A. Structure of linear tetrapyrrole chromophore biliverdin (BV) found in P2. BV is bounded in the light-sensing module that comprises of PAS (Per-ARNT-Sim), Gaf (cGMP phosphodiesterase/adenylyl cyclase/FhIA) and PHY (phytochrome) domain. Red lightinduced isomerization of the C15=C16 double bond is driving changes in the orientation of the BV and causes conformational change in the protein (2). B. Crystal structure of closley related dimeric bacteriophytochrome P3. The domains are showed in different colors. Green color, PAS domain and the red color, GAF domain. Chromophore biliverdin is bounded to Cys-28 in the GAF domain. Approximate dimensions of the protein measured using Pymol software were found to be 45 Ǻ and 68 Ǻ (3). B. kinase module Full-length N PAS GAF PHY HisKA CBD+PHY N PAS GAF PHY P2 CRYSTALS ATPase A. B. B A A B FIGURE 5. Principles of STM Operation and Experimental Design photosensory module B. A. A. Hexagonal arrangement of self-assembled monolayer of P2. Six line scans were used to measure the size of P2 to be 6.16 ± 0.17 nm. Using the adjustment factor calculated through calibration, P2 has a diameter of 6.59 nm. The image was scanned at a tunneling current of 1 nA and bias voltage of -50 mV. B. 3-D view on the protein lattice observed on the HOPG surface. B. A C. A 0.39 • Characterize the conformational changes in the structure of P2 and P3 upon red-light exposure using scanning probe techniques Pr dark (Pr) light (Pfr) B B 0.19 Future Directions Pfr • Hexagonal arrangement of the P2 protein may be driven by remarkable interaction of protein molecules with each other. STM studies of P2 in the darkadapted state will determine if this pattern is unique to the excited state and may shed light on electrostatic interactions driving the assembly. Acknowledgements FIGURE 1. -0.01 240 340 440 540 640 740 840 A. Silver-stained denaturing protein gel showing the purity of the isolated P2. After P2 synthesis was induced in E. coli cultures carrying the genes coding for P2, column chromatography was employed to separate P2 from other cellular proteins (lanes 1,3, and 4). B. Domain composition of P2, MW= 76 kD. Domain arrangement of full-lenght P2 (and P3) showing chromophore binding domain (CBD) and histidine kinase domain. Here, we imaged P2 protein containing a photosensory core, PAS, GAF and PHY domains. C. UV-visible absorption spectra of P2 (PAS-GAF-PHY) lacking histidine kinase domain. Spectra were measured in solution in the dark-adapted Pr (black) and light-illuminated Pfr (red) states. Pr, dark-adapted state peak centerd at 710 nm and Pfr, the exciated state centered at 750 nm (1). FIGURE 3. A. A probe tip is located at the corner of tripod made from piezoelectric ceramic. The purpose of the x and y piezoelectric crystals is to move the tip over the sample in the x and y direction. The z piezoelectric crystal together with the feedback control circuit is used to monitor the movement of the tip in the z direction maintaining the tunneling current constant (4). B. Calibration of STM instrument. HOPG imaged upon application of 0.20 mM NaCl. Six line scans were used to measure the lattice parameter of HOPG to be 0.23 ± 0.01 nm. Literature reports the value to be 0.246 nm. This derives a correction factor of 1.07. The HOPG images were acquired at a tunneling current of 1 nA and bias voltage of -50 mV. The STM measurements were performed at ambient temperature and pressure using the Nanosurf® easyScan 2 STM. We would like to thank the Student Center for Science Engagement at NEIU for financial support. In addition, we would like to thank Dr. Qiti Guo and Dr. Ka Yee C. Lee at The University of Chicago Materials Research Science and Engineering Center for helpful discussions and additional training on scanning probe instruments. References 1. E. A. Stojkovic. Purification and crystallization of R. palustris RpBphP2 bacteriophytochrome. Introduction to Laboratory Exercises (2007) 2. E. Giraud et al. Journal of Biological Chemistry. 280:32389-32397(2005) 3. X. Yang, E. A. Stojkovic et al. PNAS. 104:12571-12576 (2007) 4. D. A. Bonnell ed. (1993). Scanning Tunneling Microscopy and Spectroscopy. Theory, Techniques, and Applications. New York: VCH Publishers, Inc.