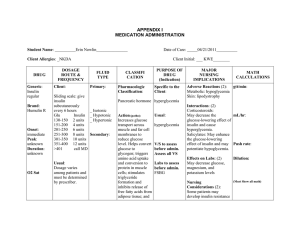
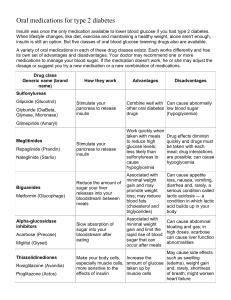
Management of Hyperglycemia
advertisement

Inpatient Glucose Control Resident’s conference Ghana Kang, R2 9/13/2011 Hyperglycemia and hospitalization A recent survey estimated that 22% of all hospital inpatient days were incurred by people with diabetes and that hospital inpatient care accounted for half of the $174 billion total US medical expenditures for this disease It’s estimated that 12-25% of patients who are in hospital beds have diabetes or some degree of hyperglycemia. Of patients in the cardiac care unit, one out of two may have diabetes or glucose intolerance. Glucose measurement Glucose Measurement Methodologies The small and colorless glucose molecule is very difficult to measure directly. Therefore, all marketed glucose measurement devices use indirect measurement methods. All techniques are enzymatic, with measurement of byproducts by optical or electrochemical methods. Byproduct formed is directly proportional to the amount of glucose present. Hexokinase: used most commonly in central laboratory devices. Glucose oxidase (GO) Glucose-1-dehydrogenase (GDH) A second GDH-based measurement system with steadily increasing market share, Glucose-1-dehydrogenase pyrroloquinolinequinone (GDH-PQQ) is more nonspecific for glucose. May yield falsely high glucose readings in the presence of maltose, xylose, or galactose Andrew D. Pitkin, et al., Challenges to Glycemic Measurement in the Perioperative and Critically Ill Patient: A Review, J Diabetes Sci Technol 2009;3(6):1270-1281 Factors affecting accuracy of glucose measurement 1. Terminology A potential error in current practice arises from the use of blood and plasma glucose as interchangeable terms, with the consequent risk of misinterpretation. The glucose concentration in plasma is approximately 11% higher than that in whole blood because plasma is denser than whole blood A mutiplier of 1.11 for the conversion of glucose in blood to plasma has been recommended. (55%) (<1%) (45%) Factors affecting accuracy of glucose measurement 1. Terminology The physiologic activity of glucose corresponds more closely with plasma concentration than whole blood glucose concentration, which varies considerably with hematocrit. Most of the POC devices measure glucose in whole blood and self correct internally, reporting results as plasma glucose. Factors affecting accuracy of glucose measurement 2. Sampling site ADA and WHO recommend the use of venous plasma glucose for measuring and reporting. The widespread use of capillary blood sampling despite evidence that this may lead to measurement error (fingertip blood samples ≈ capillary blood samples) The difference between capillary and venous glucose is typically small in non-hypotensive fasting subjects, but can be up to 8% higher in capillary blood after meals or glucose challenge. Compared to capillary blood, arterial sampling is generally accepted to be a more accurate measurement. Factors affecting accuracy of glucose measurement 3. Patient and Environmental Factors Hematocrit hematocrit glucose High oxygen tension (ie, pO2 >100 mm Hg) can falsely decrease glucose readings on some glucose oxidase-based blood glucose meters, especially when patients are receiving oxygen therapy. The enzyme GDH is less specific for glucose than glucose oxidase and but is not as susceptible to variations in oxygen concentration Andrew D. Pitkin, et al., Challenges to Glycemic Measurement in the Perioperative and Critically Ill Patient: A Review, J Diabetes Sci Technol 2009;3(6):1270-1281 FDA Public Health Notification: potentially fatal errors with GDH-PQQ glucose monitoring technology. August 13, 2009. Available at http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed August 30, 2009. Insulin Analogues History 1922 Banting and Best use bovine insulin extract on human Porcine insulin has only a single amino acid variation from the human insulin, and bovine insulin varies by three amino acids. Both are active on the human receptor with approximately the same strength. The first commercial insulin preparations contained numerous impurities and varied in potency from lot to lot by as much as 25 percent 1936 Hagedorn discovers that adding protamine to insulin prolongs the effect of insulin 1946 Nordisk formulates Isophane porcine insulin (Neutral Protamine Hagedorn or NPH insulin) by adding Neutral Protamine to regular insulin Protamine 1953 NPH Novo formulates Lente porcine and bovine insulins by adding zinc for longer-lasting insulin Zinc Lente 1981 1983 Novo Nordisk chemically and enzymatically converts porcine insulin to 'human' insulin Lilly produces synthetic, recombinant 'human' insulin, branded Humulin By the early 1980s, the development of purified pork insulin and then recombinant human insulin virtually eliminated insulin allergy and immune-mediated lipoatrophy. These achievements marked a slowdown in the innovation of insulin products until the 1990s, when the reports of the Diabetes Control and Complications Trial4 and the United Kingdom Prospective Diabetes Study5 confirmed the value of glycemic control in the delay or prevention of complications of diabetes. 1996 Lilly Humalog "lispro" insulin analogue approved by the U.S. FDA 2000 ”Aspart” Insulin analogue 2004 ”Glulisine” Insulin analogue 2003 2006 ”Glargine” Insulin analogue ”Detemir” Insulin analogue AHRQ Pub. No. 08(09)-EHC017-3 March 2009 ???Risk of cancer in patients receiving insulin analogues In spite of the clinical superiority, the potential mitogenic effect of some analogues remains to be clarified and constitutes a fundamental safety issue. Insulin and IGF-1 receptors display >50% of amino acid sequence homology and even >84% in the tyrosine kinase domain, while both ligands bind to both receptors. So far, no reliable date on this has been published. In 2000, adenocarcinoma with the use of insulin AspB10 in laboratory animals. In 2009, glargine ???cancer The FDA, ADA, AACE, and EASD have formally stated that patients should continue to use insulin analogues until more information is available. QUESTION 1: Does improving glycemic control improve clinical outcomes for inpatients with hyperglycemia? A) Yes B) No Era of unawareness A decade ago, there was a general lack of recognition of the need to identify and treat hyperglycemia among hospitalized patients. Acute hyperglycemia was often considered to be benign and even a physiological reaction to acute illness. There were no published guidelines or glycemic targets for the inpatient setting, so hyperglycemia in the hospital setting was essentially ignored. There is substantial observational evidence linking hyperglycemia in hospitalized patients (with or without diabetes) to poor outcomes Diabetes contributes to longer hospital length of stay regardless of the reason for admission. Completed Intensive Insulin Interventions Studies showing benefit Study Patient Methodolog Populations y Intervention Outcome Malmberg 1995 (DIGAMI I) AMI in diabetes Prospective, case IV insulin therapy followed control study by multiple insulin injections (126-196mg/dl) 1-year mortality 29% Van Den Berghe 2001 Surgical ICU Prospective, randomized controlled Glucose level 80110mg/dl vs. conventional tx. Mortality 34% Furnary 2003 Diabetes-CABG surgery Case/control study IV insulin maintains a glucose<200 compared with SQ insulin Mortality 50% with IV insulin Krinsley 2004 Mixed medical/surgical ICU Case/control study (historical control group) Compared preprotocol with postprotocol to maintain glucose<140 Mortality 29.3% The varying glucose target levels of these studies make it difficult to compare their results Van den Berghe G, et. al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345: 1359-1367 Prospectively evaluated 1500 patients in the surgical ICU. For the first time, patients’ BG was controlled to a very low target (80-110mg/dL) Intensive insulin therapy reduced overall in-hospital mortality by 34%, bloodstream infections by 46%, acute renal failure requiring dialysis by 41 %. In response…… Because of the dramatic reduction in mortality with normalization of glucose levels in the single center, the Van den Berghe study led to widespread adoption of this practice in ICUs worldwide. On the basis of initial studies, the American Association of Clinical Endocrinologists (AACE) convened a consensus conference and published the first inpatient glycemic targets in 2004. Tight glycemic control for the critically ill patient In 2005, for the first time, the American Diabetes Association (ADA) published recommendations for inpatient glycemic care in its yearly standards of care. Very quickly it was recognized that implementing inpatient glycemic control was not a simple task and that there were many barriers to overcome. In 2006, conference was held to address some of the barriers and make recommendations on how to overcome them. Call for creation of inpatient multidisciplinary steering committees Completed Intensive Insulin Interventions Studies showing no benefit Most recent studies have not shown a major benefit from controlling BG to a target of 80-110mg/dL. Study Patient Populations Methodology Intervention Outcome Malmberg 2005 (DIGAMI 2) AMI in diabetes Prospective, randomized, multicenter trial 3 distinct insulin strategies including IV insulin No difference in mortality Van Den Berghe 2006 Medical ICU Prospective, randomized, controlled study Glucose level 80-110mg/dl vs conventional treatment No significant decrease in mortality NICESUGAR 2009 Mixed medical/surgical ICU Prospective, randomized, controlled, multicenter trial Glucose of 81-108ml/dl vs conventional glucose<180mg/dl Mortality 2.6% in the intensive-control group Data on Blood Glucose Level, According to Treatment Group Conventional control group Target<180mg/dL Intensive control group target: 81-108mg/dL achievement of a glucose level modestly above the target range in a substantial proportion of patients in the intensive-control group. The NICE-SUGAR Study Investigators. N Engl J Med 2009;360:1283-1297 Probability of Survival and Odds Ratios for Death, According to Treatment Group hazard ratio, 1.11; 95% CI, 1.01 to 1.23; P=0.03 -The 90-day mortality was significantly higher in the intensively treated versus the conventionally treated group (78 more deaths; 27.5% versus 24.9%; P = .02) in both surgical and medical patients. -Mortality from cardiovascular causes was more common in the intensively treated group (76 more deaths; 41.6% versus 35.8%; P = .02). -Severe hypoglycemia was also more common in the intensively treated group (6.8% versus 0.5%; P<.001). The NICE-SUGAR Study Investigators. N Engl J Med 2009;360:1283-1297 Severe hypoglycemia in NICE-SUGAR study Glucose levels were measured using either Point-ofcare (POC) devices or Central laboratory devices (CLD) with samples obtained from either arterial catheters or capillary sites. The recorded number of episodes of severe hypoglycemia (defined as a blood glucose level ≤40mg/dL) was 272 in the intensive control group, as compared with 16 in the conventional-control group; 173 of all 288 episodes (60.1%) were confirmed by a laboratory measurement. Hyperglycemia in Hospitalized Medical and Surgical Patients in Non-ICU Settings No RCTs have examined the effect of intensive glycemic control on outcomes in hospitalized patients outside ICU settings. Several observational studies, however, point to a strong association between hyperglycemia and poor clinical outcomes, including prolonged hospital stay, infection, disability after discharge from the hospital, and death QUESTION 1: Does improving glycemic control improve clinical outcomes for inpatients with hyperglycemia? Overall, although a very tight glucose target (80 to 110 mg/dL) was beneficial in a predominantly surgical ICU population, this target has been difficult to achieve in subsequent studies, including NICE-SUGAR study, without increasing the risk for severe hypoglycemia. There has been no consistent reduction in mortality with intensive control of glycemia, and increased mortality was observed in the largest published study to date. The reasons for this inconsistency are not entirely clear. QUESTION 2: What glycemic targets can be recommended in hospitalized patients? A) B) C) D) Fasting <140 Random 140-180 None of the above A and B Recommendation of Glycemic targets in different patient populations in the hospital setting -Review of guidelines AACE-ADA guideline (2009) Moghissi,et.al., Consensus: Inpatient Hyperglycemia, Endocr Pract. 2009;15(No. 4) Amir Qaseem, et.al., Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: A clinical practice guideline from the American College of Physicians, Ann Intern Med. 2011; 154:260-267 ACC/AHA Guidelines (2007) “The usefulness of strict control of blood glucose concentration during the perioperative period is uncertain in patients with diabetes mellitus or acute hyperglycemia who are undergoing noncardiac surgical procedures without planned ICU admission.” “It is reasonable that blood glucose concentration be controlled during the perioperative period in patients with diabetes mellitus or acute hyperglycemia who are at high risk for myocardial ischemia or who are undergoing vascular and major noncardiac surgical procedures with planned ICU admission.” J Am Coll Cardiol 2007:50:e242 Question 2. What glycemic targets can be recommended in different patient populations in the hospital setting? All experts now seem to agree that a target of nearnormalization of blood glucose levels in hospitalized patients is inappropriate but also that hyperglycemia can have adverse consequences and should be treated in a substantial fraction of hospitalized patients. AACE/ADA guidelines ICU patients: 140-180 non-ICU patients: Fasting<140, random <180 ACP guidelines ICU patients: 140-200 There is difference of opinion revolving around the details of the specific target range for blood glucose in hospitalized patients, a subject that is evolving as the research base increases. Tailoring insulin use to the clinical scenario Sliding Scale Insulin To date, since the discovery of insulin in 1921, no study has shown a benefit of SSI in improving glycemic control or clinical outcome. “cookbook” approach to hyperglycemia Simple and convenient, avoids telephone calls to the physicians Retroactive and inefficient therapy that allows wide glycemic fluctuation, putting patients on a roller coaster of fluctuations in blood glucose. In a prospective RCT(RABBIT 2 Trial)*, it was found that basalbolus insulin resulted in lower mean fasting and random blood glucose levels in comparison with SSI alone without the use of basal insulin. *Umpierrez GE , Smiley D, Zisman A, et al., Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT2 trial), Diabetes Care. 2007; 30;2181-2186 AACE-ADA guideline (2009) Moghissi,et.al., Consensus: Inpatient Hyperglycemia, Endocr Pract. 2009;15(No. 4) Physiologic Subcutaneous Insulin Protocols Basal insulin glargine, detemir, or NPH Prevents gluconeogenesis and ketogenesis Bolus insulin (nutritional or prandial) Lispro, aspart, or regular Attempts to control the prandial glucose excursion Correction insulin (supplemental) Lispro, aspart, or regular Fine tunes suboptimal glycemic control by offering the flexibility of adding insulin beyond the calculated nutritional dose. Needs to be distinguished from SSI, which refers to an insulin regimen that is given alone with the sole purpose of “resolving” hyperglycemia and is not scheduled in combination with basal insulin. Correction or Supplemental Insulin Algorithms Blood glucose (mg/dL) Low-dose algorithm for pts taking≤0.5U/kg daily Moderate-dose algorithm for pts taking>0.5U/kg daily High-dose algorithm for pts taking>1U/kg daily Ac Hs Ac Hs Ac Hs 150-200 1 0 2 0 3 0 201-250 2 1 4 2 6 3 251-300 3 2 6 4 9 6 301-350 4 3 8 6 12 9 351-400 5 4 10 8 15 12 >400 6 5 12 10 18 15 Ariana R. Pichardo-Lowden, et.al., Management of hyperglycemia in the non-intensive care patient: Featuring subcutaneous insulin protocols, Endocrine practice vol 17 No.2 March/April 2011 Total Daily Dose (TDD) Can be determined by considering preadmission insulin needs, overall glycemic control, body weight, or intravenous insulin requirements. When a dose is selected on the basis of weight, 0.5U/kg is an adequate starting dose for most patients. 0.4U/kg for patients at risk for hypoglycemia (advanced age, hemodialysis, low body weight) or with type1 diabetes 0.7U/kg or higher for more insulin-resistant patients 1. Patients Eating (an intake of at least 50% of the scheduled meals) Basal insulin Nutritional or prandial insulin Detemir or Glargine: 50% of TDD as a single injection in the morning or bedtime 50% of TDD divided equally between meals. Supplemental or correctional insulin NPH: 50% of TDD (70% of basal dose in the morning ac and 30% ac supper or hs) Most patients 0.5U/kg (TDD) Low dose ac & hs Risk of hypoglycemia 0.4U/kg (TDD) Low dose ac & hs Insulin resistant 0.7U/kg (TDD) Moderate dose ac & hs Example A 50 y.o man with diabetes who is 180cm (71in) tall and weighs 90kg(198 lb) is admitted for pneumonia treatment with a random blood glucose level of 300mg/dL and an A1c level of 10.8%. Oral diabetic agents are discontinued, and blood glucose testing is ordered before meals and at bedtime. Calculate total daily dose of insulin 0.5U/kg X 90kg = 45 units. 50% 50% Basal insulin Nutritional or prandial insulin Supplemental or correctional insulin 23 units of insulin glargine taken once daily (50% of TDD) 7-8 units of insulin aspart (Novolog) taken zero to 15 minutes before meals (50% of TDD, in three divided doses) Based on glucose readings, give additional aspart (novolog) per standard correctional insulin schedule 2. Patients Fasting Basal insulin Nutritional or prandial insulin Detemir or Glargine: 50% of TDD as a single injection in the morning or bedtime None if entirely fasting. NPH: 50% of TDD (Divide amount in 3 equal doses to be given q 8h if GFR>50ml/min or divide in 2 equal doses to be given q 12h if GFR<50ml/min) Supplemental or correctional insulin If glucose>180mg/dL X 2 days and taking clear liquids, start 25% of TDD divided equally between meals. Most patients 0.5U/kg (TDD) Low dose q6h Risk of hypoglycemia 0.4U/kg (TDD) Low dose q6h Insulin resistant 0.7U/kg (TDD) Moderate dose q6h 3. Transition from IV to SC insulin Patients who receive IV insulin infusions will usually require transition to subcutaneously administered insulin when they begin eating regular meals or are transferred to lower intensity care. Typically, a percentage (usually 75% to 80%) of the total daily IV infusion dose is proportionately divided into basal and prandial components Amount used in last 6 hours X 4. If insulin drip rate is fluctuating >1U/h within past 6 hours, consider continuing the drip and reassess, or calculate TDD= 80% of 6 stable doses (insulin units/h) within the last 12 hours X 4. Subcutaneously administered insulin must be given 2 to 3 hours before discontinuation of IV insulin therapy in order to prevent hyperglycemia. Example 1AM 2AM 3AM 4AM 5AM 6AM 7AM 8AM 9AM 10AM 11AM Noo n 7U/h 8U/h 6U/h 5U/h 5U/h 5U/h 4U/h 4U/h 5U/h 4U/h 3U/h 2U/h Insulin requirement in the past 6 hours = 4+4+5+4+3+2= 22 units TDD =0.8 X (22 units X 4) = 70 units. Basal = 50% of TDD = 35 units Prandial= 50% of TDD in 3 divided doses=35 units/3 =12 units each meal. Specific clinical situations 4. Patients Receiving high dose steroids Hyperglycemia is a common complication of corticosteroid therapy. Several approaches have been proposed for treatment of this condition, but no published protocols or studies have investigated the efficacy of these approaches. A reasonable approach is to institute glucose monitoring for at least 48 hours in all patients receiving high-dose glucocorticoid therapy and to initiate insulin therapy as appropriate. Patients receiving high-dose steroids Corticosteroids has an effect on the overall insulin requirements several hours after their administration, and they notoriously exaggerate postprandial glucose. A strategy to address persistent hyperglycemia related to a single dose of a corticosteroid, typically given in the morning, is the initiation of NPH insulin, taking advantage of similar peak and duration effects of NPH and prednisone/prednisolone. 0.1U/kg for every 10mg of prednisone a day up to 0.4U/kg. (Clore and Thurby-Hay) Patients receiving high-dose steroids The administration of multiple high doses of glucocorticoids throughout the day. In this situation, NPH alone may fail to achieve and maintain glycemic control, usually because of postprandial hyperglycemia. Basal insulin Nutritional or prandial insulin Supplemental or correctional insulin 30% of the TDD for patients eating 70% of the TDD divided equally between meals. Moderate dose q ac & hs if eating or q 6h if NPO for taking<0.5U/kg TDD or at risk of hypoglycemia. High dose for taking>0.5U/kg TDD Patients receiving high-dose steroids During corticosteroid tapers, insulin dosing should be proactively adjusted to avoid hypoglycemia. Percentage of insulin decrease or increase should be half the percent steroid decrease or increase. (i.e., if steroid dose is reduced or increased 50%, insulin dose is reduced or increased 25% simultaneously-basal and nutritional) Specific clinical situations 5. Patients Receiving TPN The high glucose load in standard parenteral nutrition frequently results in hyperglycemia, which is associated with a higher incidence of complications and mortality in critically ill patients in the ICU. Insulin therapy is highly recommended, with glucose targets as defined previously on the basis of the severity of illness. Patients receiving TPN Basal insulin Nutritional or prandial insulin Supplemental or correctional insulin Use insulin drip X 24 h. None if entirely fasting Low dose q ac & hs if eating or q 6h if NPO for taking<0.5U/kg TDD or at risk of hypoglycemia. Calculate TDD as per IV to SQ protocols. Incorporate 80% of the new TDD as regular insulin in the TPN bag. When TPN bag containing insulin is started, discontinue insulin infusion. Daily dose adjustment: If BG>180, insulin in the TPN by 20% (most patients) or 10% if risk of hypoglycemia. May use SQ long- or intermediate-acting insulin while waiting for insulin dose adjustment in subsequent TPN bag. Initiate preprandial insulin if daytime glucose>180mg/dl X 2 days. If taking clear liquids: give 0.05U/kg ac If taking food: give 0.1U/kg ac If at risk for hypoglycemia, give 0.05U/kg ac Moderate dose for taking>0.5U/kg TDD Specific clinical situations 6. Patients Using an Insulin Pump Patients who use continuous subcutaneous insulin infusion (pump) therapy in the outpatient setting can be candidates for diabetes self-management in the hospital, provided they have the mental and physical capacity to do so. Of importance, nursing personnel must document basal rates and bolus doses on a regular basis (at least daily). The availability of hospital personnel with expertise in continuous subcutaneous insulin infusion therapy is essential Daily dose adjustment Basal insulin Nutritional or prandial insulin If fasting and morning BG>140mg/dL If eating and random/postprandial BG>180mg/dL Patients on≤0.5U/kg or *at risk for hypoglycemia By 10% By 10% Patients on >0.5U/kg By 20% By 20% If fasting and morning BG<100mg/dL X 2 or B<70mg/dL anytime If eating and random/postprandial BG<100mg/dL X 2 or B<70mg/dL anytime By 20% By 20% For all patients Supplemental or correctional insulin *at risk for hypoglycemia: insulin naïve, Elderly (>75 years), underweight-BMI<18.5kg/m2, renal dysfunction (GFR<50ml/min), severe hepatic or cardiac dysfunction, known gastroparesis (consider using regular insulin instead of lispro or Aspart) Transition to outpatient care Preparation for transition to the outpatient setting should begin at the time of hospital admission. Early assessment of a patient’s cognitive abilities, literacy level, visual acuity, dexterity, cultural context, and financial resources for acquiring outpatient diabetic supplies allows sufficient time to prepare the patient and address problem areas. Discharge planning, patient education, and clear communication with outpatient providers are critical for ensuring a safe and successful transition to outpatient glycemic management. THD Practice AS-IS THD Insulin Sliding Scale ------------------------------------------ Universal SSI in EPIC unless Glucose AC HS physicians create their own SSI. 60-120 NO insulin NO insulin No consideration of the degree of 121-150 2 units NO insulin insulin resistance. 151-200 4 units NO insuiln SSI (novolog) +/- Long acting 201-250 6 units 3 units (Lantus) 251-300 8 units 4 units Does this novolog SSI include 5 units prandial and correctional components 301-350 10 units 351-400 12 units 6 units or is it just correctional without prandial component? > 400 15 units 7 units -------------------------------------------- TO-BE?? SSI-L, SSI-M, SSI-H in order set. Prandial component based on individual patient’s TDD should be incorporated CONCLUSIONS Hyperglycemia in hospitalized patients is extremely common. Hospitalization is an opportunity to evaluate long-term diabetes control and initiate new therapy. Think about various factors that may affect accuracy of glucose measurement, if there are discrepancies between venous and capillary readings. A target of near-normalization of blood glucose levels in most hospitalized patients is inappropriate. Different targets for different patient populations. Always use your clinical judgment. Stringent targets for patients with high risk of MI, vascular and noncardiac major surgery with planned ICU admission Do more than SSI. Physiologic Insulin protocol is the preferred method. Always reassess and adjust accordingly. References 1.Ven den Berghe G, et. al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345: 1359-1367 2. Moghissi ES, Reexamining the evidence for inpatient glucose control: New recommendations for glycemic targets, Am J Health-Syst Pharm, vol 67, Aug 15, 2010 Suppl 8 3. The nice-sugar study investigators, Intensive versus Conventional Glucose Control in Critically Ill Patients, N Engl J Med Volume 360(13):1283-1297 4. Moghissi,et.al., Consensus: Inpatient Hyperglycemia, Endocr Pract. 2009;15(No. 4) 5. Amir Qaseem, et.al., Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: A clinical practice guideline from the American College of Physicians, Ann Intern Med. 2011; 154:260267 6. Ariana R.Pichardo-Lowden, et.al., Management of hyperglycemia in the non-intensive care patient: Featuring subcutaneous insulin protocols, Endocr Pract. 2011; 17 (No.2) 7. Visiliki Valla, Review article: Therapeutics of diabetes mellitus: Focus on insulin analogues and insulin pumps, Hindawi Publishing corporation Esperimental diabetes research volume 2010, article ID 178372, 14 pages 8. Leile K.Dawson and et.al., Risk of cancer in patients receiving insulin glargine, Am J Health-Syst Pharm Vol 67 Dec 1, 2010, 2025-2031 References 9. I.B. Hirsch, Insulin analogues, N Engl J Med 2005;352:174-83 10. Umpierrez GE , Smiley D, Zisman A, et al., Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT2 trial), Diabetes Care. 2007; 30;2181-2186 11. Ariana R. Pichardo-Lowden, et.al., Management of hyperglycemia in the nonintensive care patient: Featuring subcutaneous insulin protocols, Endocrine practice vol 17 No.2 March/April 2011 12. Konrad C.Nau, et.al., Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin, Americal Family Physician, volume 81, number 9, 1130-1135 13. Andrew D. Pitkin, et al., Challenges to Glycemic Measurement in the Perioperative and Critically Ill Patient: A Review, J Diabetes Sci Technol 2009;3(6):1270-1281 14. http://www.medscape.org/viewarticle/714742