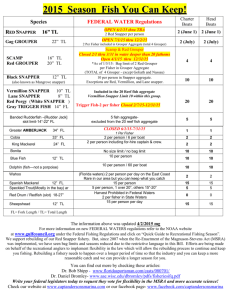
Red_Snapper_Final_Report_(Jason_Brandt)
advertisement

ASSESSMENT OF ARTIFICIAL REEF DISTRIBUTION PATTERN INFLUENCES ON RELATIVE ABUNDANCE OF JUVENILE RED SNAPPER ALONG THE MISSISSIPPI GULF COAST Final Project Report 1 August 2007 – 1 May 2010 Jason R. Brandt and Donald C. Jackson1 Department of Wildlife and Fisheries Mississippi State University Box 9690 Mississippi State, MS 39762 1 Principal investigator and to whom correspondence should be sent. ACKNOWLEDGEMENTS I would like to extend a heartfelt thanks to all of the individuals who helped with my research and thesis. First and foremost, to my major advisor Dr. Donald C. Jackson, thank you for this amazing opportunity and the support you have provided throughout my time here. Thank you to my thesis committee members Dr. Leandro (Steve) E. Miranda and Dr. Eric D. Dibble for your guidance and input during the thesis preparation and writing process. Thanks is given to the Department of Wildlife, Fisheries and Aquaculture for all of the logistical support, and to all of the department faculty and staff who helped me along the way. Special appreciation is extended to Kerwin Cuevas, Jimmy Sanders, Erik Broussard, Brandon Hall and all other members of the Mississippi Department of Marine Resources (MSDMR) who accompanied me out on the water and assisted me in the office. You all made me feel welcome, and I truly could not have accomplished my research without your help. Finally, thank you to the charter boat captains Tom Becker, Jay Trochesset, and Kenny Barhanovich for providing the use of your boats to access and sample my study site and relating invaluable personal experiences to aid in my study. ii TABLE OF CONTENTS ACKNOWLEDGEMENTS ................................................................................................ ii LIST OF TABLES ...............................................................................................................v LIST OF FIGURES .......................................................................................................... vii CHAPTER 1. INTRODUCTION ...................................................................................................1 2. METHODS ..............................................................................................................7 Study Site .................................................................................................................7 Study Design ............................................................................................................8 Data Analysis .........................................................................................................11 Catch per Unit of Effort (CPUE) ....................................................................11 Mean Length ...................................................................................................13 Species Diversity ............................................................................................13 Growth ............................................................................................................14 Environmental Variables ................................................................................14 3. RESULTS ..............................................................................................................16 Sampling ................................................................................................................16 Catch Composition.................................................................................................16 Catch per Unit of Effort (CPUE) ...........................................................................16 Mean Length ..........................................................................................................18 Species Diversity ...................................................................................................19 Tag Return .............................................................................................................19 Growth ...................................................................................................................20 Environmental Variables .......................................................................................20 Post-Capture Condition..........................................................................................21 4. DISCUSSION........................................................................................................22 Catch per Unit of Effort (CPUE) ...........................................................................23 Mean Length ..........................................................................................................26 iii Species Diversity ...................................................................................................28 Growth ...................................................................................................................28 Tag Return .............................................................................................................29 Environmental Variables .......................................................................................31 Post-Capture Condition..........................................................................................31 Conclusions ............................................................................................................32 Reef Balls versus Pyramids ...................................................................................34 LITERATURE CITED ......................................................................................................37 APPENDIX A. TOTAL NUMBER OF FISH BY SPECIES COLLECTED WITH TRAP NETS FROM SEPTEMBER 2007 THROUGH NOVEMBER 2008 AT EACH ARTIFICIAL REFF PATTERN WITHIN EACH SECTION OF ARTIFICIAL REEF SITE FH-13 LOCATED OFFSHORE OF MISSISSIPPI IN THE GULF OF MEXICO .........................63 iv LIST OF TABLES 1. Latitudinal and longitudinal coordinates for the sections of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 ....................................................................................44 2. Latitudinal and longitudinal coordinates for each artificial reef pattern and total visits to each section and artificial reef pattern in artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 .................................................................................................45 3. Number of total visits to each pattern and total number of pattern visits by season at artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 ...........................................................................46 4. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section A of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 ....................................................................................47 5. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section B of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 ....................................................................................48 6. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section C of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008 ....................................................................................49 7. Recapture data for red snapper captured with trap nets during sampling from September 2007 through November 2008 at artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico...................50 v 8. Condition and location of capture of red snapper which were released in any condition other than the best possible (Good) after being captured with trap nets from September 2007 through November 2008 at artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico ............................................................................................51 vi LIST OF FIGURES 1. Location of artificial reef site FH-13 in the northern Gulf of Mexico .....................53 2. Pyramid structures used to construct artificial reef complexes at artificial reef site FH-13 in the northern Gulf of Mexico (2a) and a trap that was used for collecting fish during sampling (2b) (Photographs provided by the Mississippi Department of Marine Resources) .........................................................................................................54 3. Artificial reef patterns deployed within each section of artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico ..........................55 4. Mean catch per unit of effort (CPUE; red snapper/trap soak-hour) by pattern with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids .............................................................................................56 5. Mean catch per unit of effort (CPUE; red snapper/trap soak-hour) by season with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November)..................................................57 6. Mean total length (mm) by pattern with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids .............................................................................................58 vii 7. Mean total length (mm) by season with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November) ....................................59 8. Red snapper length frequency distributions by reef pattern type. Length measurements are total lengths (mm). Red snapper were captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids .............................................................................................60 9. Red snapper length frequency distributions by season. Length measurements are total lengths (mm). Red snapper were captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November) ...............................................................61 10. Relationship between red snapper catch per unit of effort (CPUE; red snapper/trap soak-hour) and environmental variables (dissolved oxygen, salinity, and temperature) in the Mississippi artificial reef site FH-13, Gulf of Mexico, from September 2007 through November 2008 ....................................................................................62 viii CHAPTER 1 INTRODUCTION Red snapper, Lutjanus campechanus, are long-lived, bottom dwelling, predatory fish, capable of reaching large size, that range from Cape Hatteras, North Carolina, across the continental shelf in the Gulf of Mexico, to the Yucatan Peninsula (Patterson et al. 2001a). They support economically important recreational and commercial fisheries in the northern Gulf of Mexico (Collins et al. 1980; Allman et al. 2002, Franks et al. 2004) with an estimated annual value of $40 million (Rummer 2007). More than 95% of the total U.S. red snapper landings occur in the Gulf of Mexico (Gillet et al. 2001). Red snapper abundance has decreased by almost 90% in the northern Gulf of Mexico in the past two decades, and they are considered a highly over-exploited marine fish (Mitchell et al. 2004; Saillant and Gold 2006). In an effort to rebuild red snapper stocks, the National Marine Fisheries Service (NMFS) and the Gulf of Mexico Fishery Management Council (GMFMC) have undertaken a series of regulatory efforts aimed at restricting the direct harvest of adult red snapper and the indirect harvest of juveniles. Regulations for red snapper were first implemented by the GMFMC in November 1984 under the Reef Fishery Management Plan (RFMP) which stipulated a total allowable catch (TAC) and minimum size limits for the recreational and commercial sectors (Gillig et al. 2001; Garber et al. 2004). In 1 August 2000, the minimum recreational length for red snapper was increased from 15 to 16 inches total length (TL), but even with stringent regulations, the red snapper stock is still over-fished (SEDAR 2005), and the fishery has experienced major catch fluctuations as well as declines from historic fishery landings (Garber et al. 2004). Continued declines in red snapper stocks may be linked directly to high juvenile bycatch mortality in the shrimp trawl fishery, and controlling the mortality caused by the bycatch from the shrimp fishery is viewed as one of the most important factors in the recovery of red snapper stocks (Gillig et al. 2001; Peabody 2004; Saillant and Gold 2006; McDonough 2009). Parsons and Foster (2007) state that 90% of fishing mortality of juvenile (age-0 and age-1) red snapper comes from shrimp trawl bycatch, and Gallaway et al. (2007) found that juvenile red snapper enter the shrimp trawl fishery at 50 mm total length (TL) but do not appear to be fully vulnerable to the shrimp trawls until they are 100 mm TL or longer (Gallaway et al. 1999). Under the 1984 RFMP, bycatch reduction devices (BRDs) were required on Gulf of Mexico shrimp trawls with the hope of reducing juvenile red snapper bycatch mortality by up to 60%, which if accomplished would allow red snapper stocks to rebuild to sustainable levels (Gazey et al. 2008). Gallaway and Cole (1999), however, found that conditional survival of juvenile red snapper bycatch from trawls equipped with BRDs was around 12%, and Wells (2007) stated that juvenile red snapper excluded from trawls equipped with BRDs experience low survival due to increased predation by larger fish and marine mammals, physiological stress, and habitat displacement. 2 The bottom substrate of the continental shelf waters of the northern Gulf of Mexico consists mostly of sand and mud with little to no vertical relief, which are bottom traits conducive to shrimp trawling (Patterson and Cowan 2003; Wells and Cowan 2007). Though juvenile red snapper spend most of their first year over those sand and mud bottoms in the northern Gulf of Mexico, juvenile red snapper show increasing preference for natural and artificial habitat with vertical relief. The increasing preference for vertical structure may make artificial reefs important components of red snapper stock rehabilitation by offering juvenile red snapper which may be captured in shrimp trawls a place of preferred refuge off shrimping grounds (McDonough 2009). For the last 50 years, artificial reefs have been constructed and placed in the Gulf of Mexico with the intention of enhancing recreational and commercial fishing and rehabilitating depleted fish stocks. Artificial reef material has been placed off of the Mississippi coast since the 1960’s, and today roughly 16,000 acres of designated artificial reef sites can be found in the state’s offshore waters. Whereas substantial research has been done on artificial reefs off of Alabama (Strelcheck et al. 2005; Szedlmayer and Shipp 1994) and the oil rigs off Louisiana (Westmeyer et al. 2007; McDonough 2009), relatively little research has been done on artificial reefs off the coast of Mississippi and their possible roles in resource management (Lukens 1980; Lukens et al. 1989). Different aspects of artificial reef structure and placement must be taken into account to elicit desired management results, and various studies have examined how reef material, complexity, depth, isolation, density, height, and horizontal extension 3 relate to fish abundance and artificial reef success (Gregg 1995; Bohnsack and Sutherland 1985; Strelcheck et al. 2005; Harrera et al. 2002). With a large number of structural possibilities when choosing an artificial reef program, studies that look at different artificial reef patterns, orientations, and structural characteristics, and how they may influence each other, are necessary to elicit the best results for the rehabilitation of red snapper stocks (Gregg 1995). Reef spacing may be a particularly important consideration for artificial reef managers. Various studies have explored the effects of reef spacing on fish abundance (Bohnsack and Sutherland 1985; Sherman et al. 2002), with the intention of determining how much space is necessary between artificial reef structures to maximize benefits for desired fish species. For red snapper, the importance of reef spacing may be directly linked to the Resource Mosaic Hypothesis, which in part predicts that as reef spacing decreases, the access to soft-bottom prey around the reefs also decreases (Frazer and Lindberg 1994). Studies have shown that juvenile red snapper diets are largely composed of non-reef associated soft-bottom prey such as shrimp and crabs found around natural or artificial reef structures where the red snapper reside (McCawley and Cowan 2007; Peabody 2004). Juveniles may create intense areas of prey depletion around the reef structures (foraging haloes), with prey depletion increasing as reef spacing decreases because of greater overlap of foraging activity (Lindberg et al. 1990; Frazer and Lindberg 1994). The feeding haloes may have negative effects on abundance growth, and residence time of juvenile red snapper on artificial reefs as they may be forced to forage outside of the halo area making them more susceptible to predation (Lindberg 4 et al. 1990). Frazer and Lindberg (1994) believe that more widely spaced reefs should result in decreased halo overlap which should lead to an increase in density of potential soft-bottom prey species and red snapper foraging opportunities (Frazer and Lindberg 1994). Because the resource mosaic hypothesis has possible consequences for reef spacing, artificial reef managers need to understand whether the existence of foraging haloes should inform their decisions on possible spacing and placement of artificial reefs (McDonough 2009). Understanding critical biological characteristics of red snapper such as early life history requirements, growth rates, movements, pattern of settlement, and postsettlement site fidelity is important if they are to be the target species while dealing with the development and management of artificial reef fisheries (Gregg 1995) and answering the important question of whether or not artificial reefs aid in red snapper production and possible stock rehabilitation. Geary et al. (2007) believe that recruitment variability and year class strength of red snapper are likely determined during early life, and identifying habitats or conditions that favor survival during the nursery period is critical to management of red snapper. Larval red snapper spend approximately 26 days in the planktonic stage prior to metamorphosis and first appearance on benthic substrate (Szedlmayer and Lee 2004). Workman et al. (2002) found that juvenile red snapper reef dependency occurs within their first year, with age-0 and age-1 red snapper showing a preference for complex, high relief reef structure. Once recruited to structures, juvenile red snapper show high site fidelity and quick growth. Diamond et al. (2007) found that 96% of tagged red snapper in their 5 study that were small [< 37.9 cm (TL)], and captured in shallow water (< 40 m), stayed at their original tagging site. Juvenile red snapper take advantage of the increased food source and protection from predation that artificial reefs provide, allowing them to grow rapidly until they are about eight to ten years old (Wilson and Nieland 2001; Fischer et al. 2004; Horst 2005). These and other studies suggest that artificial reefs can play an important role in the enhancement of red snapper stocks in the northern Gulf of Mexico because (1) red snapper appear to recruit to artificial structures at an early age if the structures are present, (2) juvenile red snapper show high site fidelity on artificial structures and (3) once recruited to the structures, red snapper exhibit fast growth. Subsequently, and with these perspectives in mind, I conducted a study to determine the influence of placing artificial reef material in different patterns and the environmental parameters associated with the artificial reefs on the recruitment of juvenile red snapper to those structures in Mississippi coastal waters. 6 CHAPTER 2 METHODS Study Site The project area for this study was offshore artificial reef site Fish Haven-13 (FH-13), which is located approximately 40 kilometers (km) south of Pascagoula, Mississippi in the northern Gulf of Mexico (Figure 1). The site encompassed an approximate area of 38 km2 and ranged in depth from 20 to 26 meters (m). Artificial reef site FH-13 was split into three sections A (18 km2), B (10 km2), and C (10 km2) from north to south respectively across depth strata. Depth ranges for each section were: Section A (20-24 m), Section B (24-26 m), and Section C (26-27 m). Latitudinal and longitudinal coordinates for each section of FH-13 are given in Table 1. The bottom substrate of site FH-13 consisted mostly of sand and mud with little to no vertical relief, which is consistent with most of the continental shelf waters of the northern Gulf of Mexico (Patterson and Cowan 2003; Wells and Cowan 2007). Site FH-13 was chosen for this study because of its large size, appropriate sampling depth range, and the relatively small amount of other artificial reef material. This allowed for the placement of artificial reefs in the desired patterns and intervals necessary for the study to occur, with minimal influence from other structures. 7 Study Design Fish sampling began in September 2007 and ended in November 2008. Prior to sampling, pyramid shaped artificial reef structures with embedded stone outcroppings were placed in different pre-determined patterns within Site FH-13 (between March 6 and June 6, 2007) (Figure 2a). The artificial reef structures were composed of limestone and Coquina rock panels on cement frames. Each pyramid had a 3.7 m triangle base and measured 2.4 m in height. Approximate weight of each pyramid was 3.2 metric tons (mt). Artificial reef complexes were deployed in separate, predetermined patterns (treatments) within each section of FH-13 in a randomized complete block design; and designated pyramid dispersion (clumped versus outlier spreads), and pyramid placement intervals for horizontal positioning (30.5 m; 61.0 m; 91.4 m) from a central clump in Site FH-13. For clumped dispersion, five closely spaced pyramids (all vertically oriented) were used to constitute experimental units. Nine pyramids (all vertically oriented) were used to constitute each experimental unit for the outlier spread dispersion pattern. Within the outlier dispersion pattern, there were five pyramids clumped in a core location, and two groups of two pyramids each positioned equidistant at 30.5 m, 61.0 m, or 91.4 m from the core assemblage location. The Mississippi Department of Marine Resources (MSDMR) chose outlier distances in 100 feet (ft) increments (30.5 m=100 ft; 61.0 m=200 ft; 91.4 m=300 ft), and reef patterns will be referred to using those predetermined outlier increment numbers [clump, outlier 100 (OL100), outlier 200 (OL200), and outlier 300 (OL300)] 8 (Figure 3). One of each pattern (clump, OL100, OL200, and OL300) was located in each separate section (A, B, and C) of FH-13 (Table 2). Fish traps were used for fish collections. Traps were 0.97 m long, 0.67 m wide, and 0.64 m high (Figure 2b). Funnel mouth size for each trap measured 175 mm by 115 mm, with the smaller mouth openings biased towards collection of smaller, juvenile red snapper. Trap mesh size was 6.5 square centimeters (cm2). The traps were collapsible for easy storage and transport. Culbertson and Peter (1998) found that the use of traps reduced stress on captured fish and increased recapture rates from 8.4% to 29.1% compared to sampling by hook and line. Juvenile red snapper were collected to better understand the functional role of artificial reefs in increasing the future numbers of spawning adults which will ultimately aid in the rehabilitation of red snapper stocks. Szedlmayer and Lee (2004) found that juvenile red snapper recruit to reef habitat during their first year at a standard length (SL) of approximately 70 mm. Red snapper enter the recreational fishery at 406 mm (TL). My study addressed pre-recruit red snapper [> 70 mm and < 406 mm (TL)]. Locations for sampling were determined by randomly selecting Section A, B, or C. After the section was chosen, three artificial reef patterns within the section were randomly selected. Four traps baited with cut bait (Gulf menhaden, Brevoortia patronus) were set at each of the three artificial reef patterns and allowed to soak for two hours (soak time was pre-determined by MSDMR from pre-sampling). Two traps were dropped per pass over the reef site. All traps were set on the main clump of five central pyramids. 9 Traps were pulled by hand. To avoid excessive fish handling, successive traps were pulled only after all fish from the trap on deck were processed and back in the water. Data on environmental characteristics [dissolved oxygen (DO)(milligrams per liter; mg/L), salinity (parts per thousand; ppt), and water temperature (°C)] were collected at the surface, mid depth, and bottom of the water column at sampling sites using YSI 85 (YSI Incorporated, Yellow Springs, Ohio). If sample sites were within 0.8 km of one another, a reading was taken at a point between adjacent sampling sites. Additionally, depth (m), current direction, wind speed [kilometers/hour (km/h)], and wave height (m) were recorded. All fish collected in the traps were identified to species. For each collected fish, total, fork, and standard lengths were recorded (mm). Absolute number data collected for red snapper were used to determine catch per unit of effort (CPUE: red snapper/ trap soak hour) and length frequency distributions. Gitschlag and Renaud (1994) found that rapid retrieval of fish from depth may increase mortality due to hyperbaric trauma leading to catastrophic decompression syndrome (CDS). Subsequently, all fish collected during my sampling were vented using a hypodermic needle venting tool. Red snapper, gray triggerfish (Balistes capriscus), gag grouper (Mycteroperca microlepis), and lane snapper (Lutjanus synagris) were tagged with gray FLOY® (FLOY Tag and Manufacturing Incorporated, Seattle, Washington) t-bar anchor tags. Each tag was individually-numbered and had a phone number that could be called to report the capture of a tagged fish. Using a tagging gun, tags were inserted into the musculature just below the dorsal fin. This has been found to be the best location to 10 minimize tag loss (Diamond et al. 2007). To address possible tag loss, recaptured fish were tagged a second time with a second individually-numbered tag, but in the case of a second recapture, the fish would not be tagged a third time (no fish would have more than two individually numbered tags on it at a time). After tagging, fish were released immediately back into the water and their condition was inferred. Three condition categories were used: good (fish swam down vigorously after being placed back in the water), fair (fish oriented itself correctly but didn’t swim down immediately), and dead (fish was either dead or unresponsive once placed in the water). Data Analysis Catch per Unit of Effort (CPUE) The experimental design for this study represented a randomized complete block design, with sections (A, B, and C) being the blocks and one of each reef pattern (clump, OL100, OL200, and OL300) randomly placed within each block. During sampling, however, only three of the four patterns in the chosen section were sampled on a given trip. Thus, there was one missing value (un-sampled reef pattern) for each trip. Due to the fact that I repeatedly visited the same reefs time after time, repeated measures analysis was used, as estimates taken repeatedly on the same reefs were most likely correlated. Traditional repeated measures ANOVA does not readily allow data with missing values, however, so analysis was run using a repeated measures mixed linear model (PROC MIXED, SAS Institute Inc., Cary N.C. 2008). Days between tagging trips was the temporal repeated measure and patterns (nested 11 within sections) the subjects that were repeatedly sampled. The Kenward Rogers degrees of freedom adjustment was used to properly determine degrees of freedom for the analysis. The model used directly assessed the effect of reef pattern and season (independent variables) on CPUE (fish/hour; dependent variable) of red snapper. Normal probability plots and Shapiro-Wilk values generated from PROC UNIVARIATE (SAS Institute Inc., Cary N.C. 2008) were used to test the assumptions of normality. The CPUE data were found to be significantly nonnormal, thus a natural log transformation of the CPUE data was performed to satisfy the assumptions of normality. Sampling occurred during three seasons: spring (March, April, and May), summer (June, July, and August), and fall (September, October, and November). No sampling occurred in the winter months of December, January, and February due to poor sampling conditions, so the winter season was not considered for analysis. Section (block) was modeled as a random effect, with the goal of making the findings of the study applicable outside of the study area. Several co-variance structures were tested using restricted maximum likelihood (REML) and Akaike’s information criterion corrected for sample size (AICc). Based on model results, I used a spatial power (sp pow) covariance structure for the model. Model parameter estimates were generated using REML, and an alpha level of 0.05 was used for all analysis. In the case of significant results from the mixed model, least squares analysis (LSMEANS, SAS Institute Inc., Cary N.C. 2008) was used for pair-wise comparisons, with the significance level adjusted using the Bonferroni correction to 12 maintain the predetermined experimental error rate. Least squares mean CPUE and standard error (SE) estimates were generated and back-transformed for reporting. Mean Length Mean total lengths (TL; mm) of red snapper were compared among different reef patterns. Mean lengths were tested for normality using normal probability plots and Shapiro-Wilk values generated from PROC UNIVARIATE (SAS Institute Inc., Cary N.C. 2008). The length data were significantly non-normal, and a square root transformation was applied to normalize the data. Analysis was run using the same repeated measures mixed linear model (PROC MIXED, SAS Institute Inc., Cary N.C. 2008) as used for CPUE analysis, and the model used directly assessed the effect of reef pattern and season (independent variables) on red snapper mean TLs (dependent variables). Parameter estimates were generated in the same manner as for the above CPUE analysis, and least squares mean TL and standard error (SE) estimates were generated and back-transformed for reporting. Length frequency distributions were also developed for visual comparison analysis. Species Diversity Species diversity was calculated for each reef pattern on each trip to asses the effect of species diversity on red snapper CPUE and TL. Species diversity was calculated using the Shannon-Weiner Index: S H’= - ∑ pi ln pi I=1 13 (1) where S= the number of species in the sample and pi= the proportion of individual in species I (ni/N). Species diversity data were tested for normality using normal probability plots and Shapiro-Wilk values generated from PROC UNIVARIATE (SAS Institute Inc., Cary N.C. 2008), and the data were found to follow a normal distribution. Analysis was run using the same repeated measures mixed linear model (PROC MIXED, SAS Institute Inc., Cary N.C. 2008) as used above. Models directly assessed the effect of species diversity and species diversity*pattern interaction (independent variables) on red snapper natural log transformed CPUE and square root transformed TL (dependent variables). Growth Mark-recapture data were used to generate a growth estimate for red snapper. Growth rate (mm/d) was estimated by dividing the change in TL by days at large for each recaptured red snapper. An overall mean growth rate was determined by taking the mean of all individual recaptured red snapper growth rates. Environmental Variables Spearman (PROC CORR Spearman, SAS Institute version 9.2, 2008) productmoment correlation (r) was used to test for correlations among red snapper CPUE (fish/hour) and environmental variables measured in the bottom of the water column. As a nonparametric measure of association, Spearman correlation analysis does not require the assumption of normality to be met. For visual analysis, environmental parameter distributions were tested for normality using normal probability plots and Shapiro-Wilk values generated from 14 PROC UNIVARIATE (SAS Institute Inc., Cary N.C. 2008) and a square root transformation was applied to the dissolved oxygen data to satisfy the assumptions of normality. Scatter plots of natural log transformed CPUE versus square root transformed DO, salinity, and temperature were created for trend analysis. 15 CHAPTER 3 RESULTS Sampling Sampling for this project began September 28, 2007, and ended November 20, 2008. Twenty six (26) trips were made to FH-13 for data collection. Section B was visited most often (N=11), whereas Section A was sampled eight times and Section C was visited seven times (Table 2). Reef patterns within each section were sampled fairly evenly. The clump pattern was visited the most times (N=22), whereas the OL200 was visited the least number of times (N=17) (Table 3). Sampling throughout the seasons was fairly similar among patterns. During the period January-May, sampling was limited because of adverse weather and sea conditions. From September 2007 through May 2008, nine sampling trips were taken. From June 2008 through November 2008, 17 trips were taken. Catch Composition There were 1402 fish (21 species) captured during sampling at site FH-13 (Appendix A). Red snapper made up most of the total catch (66%; N=927), with gray triggerfish making up the next greatest percentage (10%, N=139). Lane snapper was the only other species that consisted of more than 5% of the total catch (6%, N=81). The greatest number of species were collected at the clump pattern (N=19), whereas 16 the least number of species were collected at the OL200 and OL300 patterns (N=12 each). Catch per Unit of Effort (CPUE) Catch per unit of effort (CPUE) of red snapper was determined for each pattern on every individual trip. Estimates of CPUE differed from trip to trip at each individual pattern for Section A (Table 4), Section B (Table 5), and Section C (Table 6). Results from the best fit mixed model (AICc=101.0) indicate CPUE did not vary significantly among different reef patterns (reef pattern: F3, 69.2=1.00; P=0.396), but CPUE did differ significantly among seasons (season: F2, 69.5=6.56; P=0.002). The inclusion of section as a random effect in the model did not improve model likelihood as the estimate was close to zero (estimate= 0.026, SE= 0.033). The geometric least squares parameter estimates of mean CPUE for the clump (mean CPUE= 1.22 fish/h; SE= 0.33), OL100 (mean CPUE= 1.38 fish/h; SE= 0.32), OL200 (mean CPUE= 1.57 fish/h; SE= 0.43), and OL300 (mean CPUE= 1.11 fish/h; SE= 0.28) patterns were similar (Figure 4). Bonferroni adjusted least squares pair-wise comparisons analysis indicated that mean CPUE differed significantly between the spring and summer seasons (P=0.001), but did not differ significantly between spring and fall (P=1.000) and summer and fall (P=0.175). For season, geometric least squares parameter estimates of mean CPUE was largest for summer (mean CPUE= 1.74 fish/h; SE= 0.38), whereas the spring season had the smallest estimated mean CPUE (mean CPUE= 1.06 fish/h; SE= 0.24). The estimated mean CPUE for fall (mean CPUE= 1.19 fish/h; 17 SE= 0.73) did not differ significantly from the spring and summer mean CPUE estimates (Figure 5). Mean Length The mean total length of red snapper collected in this study was 225.38 mm (SE= 2.24). Of the 927 red snapper captured, only 18 exceeded the legal length limit of 406 mm TL. Results from the mixed model (AICc= 231.8) indicated that mean TL differed among the four patterns (F3, 25.2= 5.39; P= 0.005) and among the seasons (F2, 42.6= 6.22; P= 0.004). The inclusion of section as a random effect in the model did not improve model likelihood as the estimate was close to zero (estimate= 0.593, SE= 0.70). Bonferroni adjusted least squares pair-wise comparisons analysis indicated that mean TL differed significantly between the OL200 pattern and the clump (P= 0.003) pattern (Figure 6). No other comparison of mean TL between patterns yielded significant results (P > 0.05). Geometric least squares parameter estimates of mean TL indicated that the OL200 pattern had the largest mean TL (mean TL= 253.16 mm, SE= 17.44) whereas the clump pattern had the smallest mean TL (mean TL= 203.54 mm, SE=16.71). The OL100 pattern (mean TL= 231.47 mm, SE= 16.43) and OL300 pattern (mean TL= 224.59 mm, SE= 17.55) had similar mean TL (Figure 6). Results from the Bonferroni adjusted least squares pair-wise comparisons analysis indicated that mean TL differed significantly between fall and spring (P= 0.029) and fall and summer (P= 0.004), but not spring and summer (P= 1.000) (Figure 7). Geometric least squares parameter estimates of mean TL indicated that the fall season had the largest mean TL (mean TL= 251.06 mm, SE= 18.30). The 18 mean TL for spring (mean TL= 219.12 mm, SE= 17.07) and summer (mean TL= 214.21 mm, SE= 13.83) did not significantly differ (Figure 7). Red snapper length frequency distributions by pattern and season show that most captured red snapper were between 125-275 mm (TL) (Figure 8 and Figure 9, respectively). Species Diversity Results from the mixed model analysis (AICc= 101.3) indicated that H’ (F1,69.4= 0.81; P= 0.371) and the H’*pattern interaction (F3,65.8= 0.42; P= 0.737) did not significantly affect red snapper CPUE. The inclusion of section as a random variable did not increase model likelihood as the parameter estimate was close to zero (estimate= 0.020, SE= 0.027). For TL, results from the mixed model analysis (AICc= 243.9) indicated that H’ (F1,62= 0.05; P= 0.823) and the H’*pattern interaction (F3,39.8= 2.42; P= 0.081) did not significantly affect red snapper TL. Including section as a random variable did not increase model likelihood as the parameter estimate was close to zero (estimate= 0.735, SE= 0.910). Tag Return I tagged a total of 852 red snapper. The discrepancy between total red snapper captured (927) and the total number of red snapper tagged (852) was due to a sampling trip October 26, 2007 when no tagging gun was brought on the trip, and a sampling trip April 22, 2008 when both tagging guns broke. Thirty one (31) red snapper were recaptured (Table 7). Two red snapper were recaptured twice, but one had lost its original tag. Overall I had a tag return rate of 4% for red snapper. Only 19 one red snapper was recaptured at a site other than its original site of tagging. It was originally tagged at the OL100 pattern in Section B and was recaptured at the OL200 pattern in Section B. That gives a 97% site fidelity estimate for recaptured red snapper (N=31). One red snapper was at large for 256 days before recapture (Table 7). Including that recapture, the average time at large for recaptures was 36 days. Excluding that particular specimen, the average time at large for red snapper recaptured during sampling was 21 days. Growth The change in TL for recaptured red snapper was divided by the days at liberty to get an estimate of the mean growth rate of recaptured red snapper. The growth rate estimate for recaptured red snapper was 0.29 mm/day (SE= 0.04; N=31) (Table 7). Looking at growth rates for individual patterns, the OL200 pattern produced the largest mean growth rate [mean= 0.47 mm/d (TL); SE= 0.08; N= 11], followed by the clump [mean= 0.23 mm/d (TL); SE= 0.04; N= 11], and OL100 [mean= 0.18 mm/d (TL); SE= 0.04; N= 9] patterns. Only one recapture came from the OL300 pattern, and that fish’s growth rate was 0.05 mm/d (TL). Environmental Variables Results from the Spearman correlation analysis indicated no significant correlations between red snapper CPUE and salinity (Spearman r = -0.13, P= 0.32), dissolved oxygen (Spearman r = -0.07, P= 0.58) or temperature (Spearman r = 0.12, P= 0.35). Scatter plots of natural log transformed CPUE versus salinity (R2= 0.006), 20 square root transformed dissolved oxygen (R2= 0.004), and temperature (R2= 0.027) revealed no strong patterns (Figure 10). Post-Capture Condition Eight hundred and ninety eight of the captured red snapper were considered to be in “Good” condition after release, 24 red snapper were considered to be in “Fair” condition after release, and five red snapper were considered to be “Dead” after release (Table 8). Of the 29 red snapper determined to be in “Fair” condition or “Dead”, only 22 were tagged, which gave an estimated acute mortality rate of 3% due to the tagging process for red snapper in my study. 21 CHAPTER 4 DISCUSSION My study was developed to examine the effects of reef spacing and horizontal extension on juvenile red snapper, with the hope of finding specific spatial patterns that would most benefit juvenile snapper and in turn aid in stock enhancement. Though there have been studies that have examined artificial reef structural characteristics and placement strategies with respect to maximum fisheries benefits for artificial reef programs (Gregg 1995; Harrera et al. 2002; Strelcheck et al. 2005), few studies have specifically examined the importance of reef spacing and placement for red snapper management. The limited information on these important reef aspects belies the importance of my study. In this regard, my focus on juvenile red snapper habitat preference is particularly important because recruitment variability and year class strength of red snapper are most likely determined during early life stages (Geary et al. 2007). The significance of juvenile survival makes identifying habitats or conditions that favor survival during early life stages critical to management. Results from this study indicate that juvenile red snapper are recruiting to the artificial reef structures that I studied. Ninety-eight percent of the red snapper caught during my study (909 fish) were under the legal recreational length limit of 406 mm (TL), and the mean length of red snapper captured was 225 mm (TL). Wells and 22 Cowan (2007) found that juvenile red snapper recruit to high relief structures at 20 cm (TL), or at age-1, and Nieland and Wilson (2003) found that juvenile red snapper disappear from shrimp trawls at age-1, migrating to higher relief structures to seek refuge from predators. Because juvenile red snapper in my study area utilized the artificial reef structures, the reefs must be providing some benefit for these fish, possibly as refuge from predation or in terms of increased foraging opportunities. Perhaps the most significant function of the artificial reefs in my study is their use as refuge for juvenile red snapper from shrimp trawls, as reduction in red snapper bycatch mortality from shrimp trawling could play a key role in red snapper stock rehabilitation (Peabody 2004). Gallaway et al. (1999) found that juvenile red snapper reach full vulnerability to shrimp trawls around 100 mm (TL). Because many of the red snapper that I captured were between 100-200 mm (TL), the artificial reefs in my study area could be providing important refuge for juvenile red snapper that would otherwise be lost as bycatch in shrimp trawls. Catch per Unit of Effort (CPUE) Results from the mixed model analysis indicated that reef pattern did not significantly affect red snapper CPUE, as all mean pattern CPUE estimates differed by < 1 fish/hour. As the primary objective of this study, a lack of significant pattern affects on red snapper relative abundance was unexpected. A variety of factors may have led to this result. Difficulties related to setting traps on, or in close proximity to, the sampled reef patterns, even when taking into account important factors such as currents and wave action, may have played a prominent role in my inability to find significant 23 pattern effects on red snapper relative abundance. The ever changing resting place of traps in proximity to the sampled patterns may have led to the large observed fluctuations in CPUE within patterns from trip to trip, and among traps set on a given reef. Different robust estimates of scale such as interquartile range and median absolute deviation from the median were examined to determine an appropriate estimate of variation from trip to trip and among traps, that could be used in significance testing. However, my small sample size and number of traps used did not work well with the robust estimates that were examined, and results generated from those estimators were misleading. Reef populations that were not yet in equilibrium may have also lead to my inability to decipher reef pattern effects on CPUE. Bohnsack and Sutherland (1985) stated that reef fish populations may reach maximum population size within a few months after the reefs are placed in the environment, and equilibrium community structure is usually achieved within one to five years. Sampling for this study began about three months after the last study reef was placed in the study area, and all sampling occurred within a year and a half of the final placement of the study reefs. I believe that red snapper which had recruited to my study reefs were still in the process of reaching maximum population size and equilibrium population structure. If the populations were not yet at a sustainable maximum size and new juvenile red snapper were recruiting continuously to the structures during the period of my study, then the use of CPUE as an index of relative abundance may have given biased results which in turn would make it difficult to determine statistically significant differences in red snapper relative abundance among the different reef patterns. 24 Though there was no significant influence of pattern on CPUE, relationships from my data indicated that the OL200 pattern produced the largest CPUE of juvenile red snapper, which is similar to the findings of Frazer and Lindberg (1994). They looked at different reef spacing of similar sized prefabricated concrete reefs and found widely spaced reef units (60 m) had a larger abundance of gray triggerfish and black sea bass (Centropristis striata) than closely spaced units (2 m), which may have been due to density dependent interactions specifically linked to foraging area and prey resources. The importance of reef spacing may be directly linked to red snapper foraging strategies and the principles of the Optimal Foraging Theory and Resource Mosaic Hypothesis (McCawley 2003). Juvenile red snapper diets are largely comprised of soft bottom prey resources. As the distance between reef units decreases so too does the access to soft bottom prey around the reef and areas of intense depletion called foraging halos develop (McCawley and Cowan 2007). If reefs are placed too close together, their associated foraging halos may overlap and negatively affect one another by causing a disproportional depletion of resources. Red snapper associated with closely spaced reefs may be forced to travel farther from the reef to forage at increased energetic cost which in turn increases the risk of predation and decreases the probability that those snapper will return to the reef leading to potential declines in abundance (McDonough 2009; Westmeyer et al. 2007; Frazer and Lindberg 1994). In this regard, the OL200 pattern may provide an adequate amount of spacing to minimize halo overlap and in turn may be able to support a greater abundance of red snapper. Though the OL300 pattern has wider spacing and in theory even less 25 foraging overlap, it is possible that the outliers of the OL300 pattern are far enough from the main clump that juvenile red snapper that venture within proximity of the outliers may decide to stay instead of risk predation traveling back to the main clump. With the closer proximity of the outlier structures of the OL200 pattern, juvenile red snapper may be more willing to move freely between the main clumps and outliers utilizing the extra structure for refuge from predation and areas of additional foraging opportunities. As expected, CPUE of juvenile red snapper differed significantly among seasons. For my study the summer season produced the largest CPUE, which runs counter to previous studies. Strelcheck (2001) and Patterson (1999) found CPUE of red snapper to decrease during spring and summer, and increase in the fall. However, both of these studies involved red snapper of larger mean size than those in my study. The presence of larger red snapper on my study reefs during the fall season may be one reason for my observed seasonal differences in CPUE. Bailey (1995) found that the presence of larger sub-adult red snapper (360-367 mm) negatively influenced the presence of young of the year red snapper by limiting refuge and foraging opportunities. For my study the fall season had the largest mean TL, and the presence of larger red snapper during the fall and early spring may have had a negative effect on juvenile red snapper CPUE. Mean Length Results from the mixed model analysis indicated that mean TL of red snapper differed significantly among patterns, with the OL200 pattern having the largest estimated mean TL. Significant differences in mean TL between patterns may be an 26 indication of increased benefits (foraging opportunities, prey abundance) specific to pattern type or reef location. Powers et al. (2003) and Wells (2007) found that larger sizes of individual fish at particular reefs may be an indication of increased refuge from predation and an increased access to reef associated prey resources, which in turn may lead to an increase in production by enhancing growth and protection of individuals utilizing the reefs. Similar reasons to those explained for my CPUE results apply in terms of why red snapper associated with the OL200 pattern had significantly larger mean TL. The OL200 design may offer the appropriate reef spacing for juvenile red snapper which in turn may increase prey access and foraging opportunities while minimizing the risk of predation. The greater distance between the main clump and outliers of the OL300 patterns may be too large for juvenile red snapper to move between freely, and the energetic costs associated with increased forage searching time and predator avoidance may have lead to slower growth rates and smaller mean TL. Red snapper mean TL differed significantly among seasons, with the fall season producing the largest mean TL. The collection of larger juvenile red snapper in the fall seems reasonable as the fish had more time to grow, but as mentioned earlier, the larger mean TL for the fall season may be a result of the movement of larger red snapper onto the artificial reefs used during my study. Wells (2007) found that seasonal size differences at specific reef habitats may likely be a result of seasonal emigration and immigration of different size groups of red snapper. The possible movement of larger fish onto my study reefs in the fall could account for the 27 larger mean total length and smaller mean CPUE of red snapper during the fall season. Species Diversity The analysis of species diversity indicated that H’ and H’*pattern interaction did not significantly affect red snapper CPUE or TL. It is important to point out that the traps used were selective for certain sizes of fish and species that would be attracted by cut-bait. As such, the diversity I observed may not be representative of the true species diversity of my sample reefs. A variety of collection gear and visual analysis would be needed to gain truly accurate species diversity estimates. Additionally, as I mentioned before, the community structures of my study reefs may had not reached equilibrium during my study period which could affect analysis, or the species that I observed from sampling may occupy different niches and not significantly affect red snapper relative abundance. Growth The mean growth rate for red snapper during my study (0.29 mm/day) indicates similar growth to previous studies of red snapper. Patterson et al. (2001b) found the growth rate of tagged red snapper to be 0.24 mm/day (TL), although the mean length of red snapper in their study was larger (mean= 335 mm TL) than the mean length of red snapper in my study. Watterson (1998) found that fish tagged at initial lengths less than 300 mm (TL) exhibited a larger growth rate of 0.36 mm/day compared to 0.23 mm/day for red snapper between 300-399 mm. 28 The OL200 pattern had the fastest mean growth rate among the patterns. Although sample sizes were not large enough for statistical tests, the faster growth rate for the OL200 pattern may be another indication that the OL200 pattern design offers significant benefits to the juvenile red snapper in the study area, which in turn may lead to increased production through increased abundance and biomass (Powers et al. 2003 and Wells 2007). If red snapper that utilize the OL200 patterns are able to translate added benefits into quicker growth, they would be able to move quickly out of vulnerable juvenile stages and avoid predation (Wells 2007). The faster estimated growth rate from the OL200 recaptures may offer some explanation as to why I saw the significantly larger mean TL for that pattern as well. Tag Return The site fidelity estimate for recaptured red snapper in this study was 97%, which falls in line with other studies involving juvenile red snapper. Strelcheck et al. (2007) and Workman et al. (2002) found that juvenile red snapper exhibit high site fidelity, and homing abilities when displaced from structure. Diamond et al. (2007) found that 96% of tagged red snapper in their study that were small [< 379 mm (TL)] and captured in shallow water (< 40 m) stayed at their original tagging site, which is very similar to my study as all of my recaptured red snapper were < 379 mm (TL) and captured in water < 40 m deep. Diamond et al. (2007) believe that reef fish taken from shallower water (< 30 m) move less than fish taken from deeper water, most likely because shallow water generally has higher productivity. One red snapper in my study was recaptured at a site other than that of its original tagging, and that fish moved from the OL100 pattern in Section B to the 29 OL200 pattern in Section B. This provides further evidence for the suitability of the OL200 pattern for juvenile red snapper, as high site fidelity may be a strong indication of habitat suitability (McDonough 2009). I recorded a tag return rate of 4% for red snapper, which is similar to results from other studies. Diamond et al. (2007) found the tag return rate for red snapper in their study to be 2.3%, but Patterson (2007) found that tag return rates for red snapper studies have varied between 2.8-35%. I noticed from recaptured fish that considerable bio-fouling of tags occurred after relatively short periods of time at large, making the tag hard to see without careful examination. It is possible that some captured undersized red snapper were quickly released by fishermen without them noticing tags, which may be why I have not had any reported tag returns outside of what I collected. High rates of tag loss may have also been a direct factor in the low tag return rate. A double-tagging study of striped bass Morone saxatilis found t-bar anchor tag retention to be as low as 42% after just one year (Dunning et al. 1987). Muoneke (1992) found the rate of t-bar anchor tag loss for white bass (Morone chrysops) to be 24.8%. Of the two red snapper that were recaptured twice (double tagged) in my study, one had lost its first tag which may be a strong indication of low tag retention. On top of tag loss, another drawback to tagging with t-bar anchor tags is that even if fish show high site fidelity, it is impossible to know if or how the fish moved between capture and recapture (Watterson 1998; Szedlmayer and Schroepfer 2005). Recaptured fish in my study may have moved off of the reefs and returned, and fish that were not recaptured may have undertaken extensive movements. With no 30 information from the tagged fish that were not recaptured, my site fidelity estimate must be viewed with caution and strict understanding that the estimate is only for those fish that were recaptured. Environmental Variables No significant correlations between juvenile red snapper CPUE and measured environmental variables were observed which is similar to the study of Strelcheck (2001), as he did not find significant correlations between red snapper CPUE and temperature or red snapper CPUE and DO. Environmental variables measured during my study period may not have differed enough from trip to trip to produce a significant effect on red snapper CPUE. Interestingly, my largest single estimated trip CPUE occurred with a bottom DO measurement of 1.94 mg/L, which is well below the preferred DO level of 5.0 mg/L for juvenile red snapper determined by Gallaway and Cole (1999). Measurement error associated with the YSI meter may explain this observation, or juvenile red snapper in my study area with limited refuge options may tolerate adverse environmental conditions to avoid the risk of predation. Post-Capture Condition Acute mortality of juvenile red snapper from the tagging process for this study was fairly low. Patterson et al. (2001a) looked at acute mortality in captured red snapper due to the tagging process. For their study, they created four condition categories and assumed that fish released in any condition other than the best possible suffered acute mortality as result of the tagging process. In their study, 14% of the tagged red snapper were released in a condition other than the best possible and were 31 assumed to have suffered acute mortality from the tagging process. Following similar guidelines, I found a smaller acute mortality of 3% due to the tagging process for my captured red snapper. All recaptured red snapper in my study were originally released in the best possible condition (condition 1), which gives some credence to the use of that particular grading system as an index for acute tagging mortality. The relatively small mortality rate from the tagging process was promising, as there was some concern as to how well the smaller, pre-recruit red snapper would handle tagging. It appears that my use of t-bar anchor tags did not significantly affect release mortality of captured red snapper. Gitschlag and Renaud (1994) looked at survival rates of red snapper [25-43 cm (FL)] captured at different depths, and found 90% of the red snapper collected from depths between 27-30 meters (the depth range covering our study area) survived the retrieval process, and size of fish did not influence mortality. The slow retrieval of the traps by hand and relatively shallow sampling depths in my study may have been beneficial in terms of survival of captured fish. Conclusions Findings of this study are significant and promising, as few studies have looked at the importance of spacing and horizontal extension as they pertain to the recruitment of juvenile red snapper to artificial reef structures and their retention on those structures. Juvenile red snapper are recruiting to the pyramid shaped artificial reef structures, which may lead to a decrease in juvenile red snapper bycatch mortality as the fish move off of the shrimp grounds and onto the reefs for refuge. The rapid colonization of the artificial reef structures gives a strong indication that the 32 reefs are offering benefits to the fitness of these important reef fish species, whether it be increased shelter from predation or increased foraging opportunities. Though no significant differences were found in mean CPUE of red snapper by pattern, results from my data indicate that the OL200 pattern had the largest mean CPUE. The largest mean TL of collected red snapper also came from the OL200 pattern, which may indicate energetic benefits related to the specific reef spacing of that pattern. The faster mean growth rate of recaptured red snapper from OL200 pattern, and the fact that the only recapture that moved relocated to an OL200 pattern gives further evidence to some benefit or benefits that juvenile red snapper are able to exploit that is/are not found in the other three experimental patterns. Continued research of red snapper on my study reefs that examines important physiological and ecological aspects such as diet, prey availability, and interactions with other species could help address some of the questions my study was unable to answer. Particularly, why the mean TL and growth rate for red snapper at the OL200 patterns is larger than for the other patterns I tested. Reef spacing is just one physical component of artificial reef complexes that may affect red snapper and other reef fish recruitment to the structures. The consequences of resource depletion caused by overlapping foraging halos are a critical reason why management of artificial reefs should consider reef spacing to minimize halo overlap. Though initial costs may be higher, my results reflect the importance of species specific studies that analyze specific habitat preferences and what characteristics of those habitats that are most important to the chosen species. For artificial reefs to best aid in the rehabilitation of depleted red snapper stocks, 33 continued analysis of artificial reef physical characteristics that best benefit different life history stages must be undertaken. The results of this study provide an important and informative first step towards the understanding of the relationship between juvenile red snapper and artificial reefs off the coast of Mississippi, which will hopefully aid in the rehabilitation of red snapper stocks Gulf wide. Reef Balls versus Pyramids I was given additional information from a new project initiated by the Artificial Reef Bureau of the MSDMR looking at differences in prefabricated artificial reef materials. As artificial reefs become more prevalent in marine systems management, so too will the options of prefabricated materials. Studies that examine the effects of different prefabricated reef material on target fish species will be necessary to help managers best elicit desired results. This newly initiated study by the MSDMR is the first step towards better understanding the different effects that pyramid shaped concrete and limestone structures and concrete Goliath Reef Balls (GRB) have on red snapper and gray triggerfish CPUE and TL. For this study, pyramid shaped artificial structures and Goliath Reef Balls were placed in designated artificial reef sites FH-1 and FH-2, which are both located in close proximity to my study site FH-13 or about 40 km south of Pascagoula, Mississippi. Size specifications for the pyramid structures can be found in the methods section of this report. Goliath Reef Balls are composed of concrete, measure 1.83 m wide by 1.52 m tall, and weigh about 2.27 mt. Pyramid structures and GRBs were placed in clumps of 10 structures throughout FH-2 and FH-1. Three sets each of 10 structures were placed in each reef site. 34 Sampling took place in a similar manner as sampling for my study. Identical trap nets as used for my study were used to collect fish. After a sample reef was selected, three traps were set on the clump of pyramids or GRBs and allowed to soak for two hours. After soaking for two hours, the traps were collected one at a time by hand, and the contents of each trap enumerated. Red snapper, gray triggerfish, gag grouper, and lane snapper were tagged with gray FLOY® (FLOY Tag and Manufacturing Incorporated, Seattle, Washington) t-bar anchor tags, and each fish was measured for fork and total lengths. Statistical analysis was performed on the preliminary data. The effects of the two different materials on red snapper and gray triggerfish relative abundance and TL were examined. Catch per unit of effort (CPUE) was used as an index of relative abundance. Red snapper and gray triggerfish CPUE (fish/hour) and TL (mm) data were tested for normality using normal probability plots and Shapiro-Wilk values generated from PROC UNIVARIATE (SAS Institute Inc., Cary N.C. 2008). The data were found to follow normal distributions. Analysis was run using general linear models (PROC GLM, SAS Institute Inc., Cary N.C. 2008). Models directly assessed the effect of reef type (independent variable) on CPUE and TL (dependent variables). An alpha level of 0.05 was used for analysis. To date, the pyramid structures were visited eight times and the GRBs were visited four times (12 total visits). A total of 327 red snapper and 20 gray triggerfish were collected, with 100 red snapper and seven gray triggerfish collected from GRBs and 227 red snapper and 13 gray triggerfish collected from pyramid structures. Statistical analysis indicated that reef type did not significantly affect red snapper 35 mean CPUE (F= 0.09, P= 0.776), but reef type did have a significant effect on red snapper mean TL (F= 9.12, P= 0.0145). Red snapper mean CPUE estimates for pyramids (mean CPUE= 4.728 fish/hour, SE= 1.180) and GRBs (mean CPUE= 4.166 fish/hour, SE= 1.32) were similar, but red snapper mean TL estimates for pyramids (mean TL= 282.168 mm, SE= 9.259) and GRBs (mean TL= 242.689 mm, SE= 5.111) differed by 40 mm. For gray triggerfish, statistical analysis indicated that gray triggerfish mean CPUE (F= 0.33, P= 0.579) and mean TL (F= 0.17, P= 0.692) were not significantly affected by reef type. Gray triggerfish mean CPUE estimates for pyramids (mean CPUE= 0.208, SE= 0.087) and GRBs (mean CPUE= 0.291, SE= 0.104) were similar, and mean TL estimates for pyramids (mean TL= 281.066, SE= 16.981) and GRBs (mean TL= 261.833, SE= 55.352) differed by approximately 20 mm. As preliminary analysis, these results must be taken with caution. Statistical analysis and mean estimates were drawn off of a small sample size (12 samples), and the GRBs were visited half as many times as the pyramids. With unbalanced data, statistical results can be misleading. As the only significant result, it does appear that pyramids have larger red snapper which may be a result of faster growth or the use of the pyramids by larger, older red snapper. Further research will give a better understanding of the effects of the two different reef materials on red snapper, gray triggerfish, and other important bycatch species CPUE and TL, and provide crucial information on an important artificial reef characteristic. 36 LITERATURE CITED Allman, R. J., L. A. Lombardi-Carlson, G. R. Fitzhugh, and W. A. Fable. 2002. Age structure of red snapper (Lutjanus campechanus) in the Gulf of Mexico by fishing mode and region. Gulf and Caribbean Fisheries Institute Annual Proceedings 53:482-495. Bailey, H. K., IV. 1995. Potential interactive effects of habitat complexity and subadults on young-of-the-year red snapper (Lutjanus campechanus) behavior. Master’s thesis. University of South Alabama, Mobile, Alabama, 73 pp. Bohnsack, J. A., and D. L. Sutherland. 1985. Artificial reef research: a review with recommendations for future priorities. Bulletin of Marine Science 37(1):11-39. Collins, L. A., J. H. Finucane, and L. E. Barger. 1980. Description of larval and juvenile red snapper, Lutjanus campechanus. Fishery Bulletin 77(4):965-974. Culbertson, J. C., and D. D. Peter. 1998. Development of tagging techniques for monitoring fish populations at Texas artificial reefs. Gulf of Mexico Science 16:46-53. Diamond, S. L., M. D. Campbell, D. Olson, Y. Wang, J. Zeplin, and S. Qualia. 2007. Movers and stayers: individual variability in site fidelity and movements of red snapper off Texas. Pages 163-187 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Dunning, D. J., O. E. Ross, J. R. Waldman, and M. T. Mattson. 1987. Tag retention by, and tagging mortality of, Hudson River striped bass. North American Journals of Fisheries Management 7:535-538. Fischer, A. J., M. S. Baker, and C.A. Wilson. 2004. Red snapper (Lutjanus campechanus) demographic structure in the northern Gulf of Mexico based on spatial patterns in growth rates and morphometrics. Fishery Bulletin 102(4):593-603. 37 Franks, J. S., J. R. Hendon, and N. M. Garber. 2004. Red snapper (Lutjanus campechanus) associated with a small artificial structure in the Mississippi Sound, a northern Gulf of Mexico estuary. Gulf and Caribbean Fisheries Institute Annual Proceedings 55:853-864. Frazer, T. K., and W. J. Lindberg. 1994. Refuge spacing similarly affects reefassociated species from three phyla. Bulletin of Marine Science 55:388-400. Gazey, W. J., B. J. Gallaway, J. G. Cole, and D. A. Fournier. 2008. Age composition, growth, and density-dependent mortality in juvenile red snapper estimated from observer data from the Gulf of Mexico penaeid shrimp fishery. North American Journal of Fisheries Management 28:1828-1842. Gallaway, B. J., W. J. Gazey, J. G. Cole, and R. G. Fechhelm. 2007. Estimation of potential impacts from offshore liquefied natural gas terminals on red snapper and red drum fisheries in the Gulf of Mexico: an alternative approach. Transactions of the American Fisheries Society 136:655-677. Gallaway, B. J., and J. C. Cole. 1999. Reduction of juvenile red snapper bycatch in the United States Gulf of Mexico shrimp trawl fishery. North American Journal of Fisheries Management 19:342-355. Gallaway, B. J., J. G. Cole, R. Meyer, and P. Roscigno. 1999. Delineation of essential habitat for juvenile red snapper in the northwestern Gulf of Mexico. Transactions of the American Fisheries Society 128:713-726. Garber, A. F., M. D. Tringali, and K. C. Stuck. 2004. Population structure and variation in red snapper (Lutjanus campechanus) from the Gulf of Mexico and Atlantic Coast of Florida as determined from mitochondrial DNA control region sequence. Marine Biotechnology 6:175-185. Geary, B. W., J. J. Mikulas, Jr., J. R. Rooker, A. M Landry, Jr., and T. M. Dallapenna. 2007. Patterns of habitat use by newly settled red snapper in the northwestern Gulf of Mexico. Pages 25-38 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Gillig, D., W. L. Griffin, and T. Ozuna Jr. 2001. A bioeconomic assessment of Gulf of Mexico red snapper management policies. Transactions of the American Fisheries Society 130:117-129. Gitschlag, G. R., and M. L. Renaud. 1994. Field experiments on survival rates of caged and released red snapper. North American Journal of Fisheries Management 14:131-136. 38 Gregg, K. L. 1995. Comparisons of three manufactured artificial reef units in Onslow Bay, North Carolina. North American Journal of Fisheries Management 15:316-324. Harrera, R., F. Espino, M. Garrido, and R. J. Haroun. 2002. Observation on fish colonization and predation on two artificial reefs in the Canary Islands. ICES Journal of Marine Science 59:S69-S73. Horst, J. 2005. Red snapper fact sheet. Sea Grant Abstracts 20(1-2):25. Lindberg, W. J., T. K. Frazer, and G. R. Stanton. 1990. Population effects of refuge dispersion for adult stone crabs (Xanthidae, Menippe). Marine Ecology Progress Series 66:239-249. Lukens, R. R. 1980. The succession of ichthyofauna on new artificial reefs in the northern Gulf of Mexico. Master’s thesis. University of Southern Mississippi, Hattiesburg, Mississippi, 32 pp. Lukens, R.R., J. D. Cirino, J. A Ballard, and G. Geddes. 1989. Two methods of monitoring and assessment of artificial reef materials. Special Report 2-WB. Gulf States Marine Fisheries Commission, Ocean Springs, Mississippi, 58 pp. McCawley, J. R. 2003. Diet and prey demand of red snapper, Lutjanus campechanus, on Alabama artificial reefs. Master’s thesis. University of South Alabama, Mobile, Alabama, 205 pp. McCawley, J. R., and J. H. Cowan, Jr. 2007. Seasonal and size specific diet and prey demand of red snapper on Alabama artificial reefs. Pages 77-104 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. McDonough, M. 2009. Oil platforms and red snapper movement and behavior. Master’s thesis. Louisiana State University, Baton Rouge, Louisiana, 76 pp. Muoneke, M. I. 1992. Loss of Floy anchor tags from white bass. North American Journal of Fisheries Management 12:819-824. Mitchell, K. M., T. Henwood, G. R. Fitzhugh, and R. J. Allman. 2004. Distribution, abundance, and age structure of red snapper (Lutjanus campechanus) caught on research longlines in the U.S. Gulf of Mexico. Gulf of Mexico Science 22(2):164-172. 39 Nieland, D. L., and C. A. Wilson. 2003. Red snapper recruitment to and disappearance from oil and gas platforms in the northern Gulf of Mexico. Pages 73-81 in D. R. Stanley and A. Scarborough-Bull, editors. Fisheries, reefs, and offshore development. American Fisheries Society Symposium 36. Bethesda, Maryland. Parsons, G. R., and D. G. Foster. 2007. Swimming performance and behavior of red snapper: their application to bycatch reduction. Pages 59-75 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Patterson, W. F. 1999. Aspects of the population ecology of red snapper, Lutjanus campechanus, in an artificial reef area off Alabama. Doctoral dissertation. University of South Alabama, Mobile, Alabama, 164 pp. Patterson, W. F. 2007. A review of movement in Gulf of Mexico red snapper: implications for population structure. Pages 245-261 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Patterson, W. F., J. H. Cowan, C. A. Wilson, and R. L. Shipp. 2001a. Age and growth of red snapper, Lutjanus campechanus, from an artificial reef area off Alabama in the northern Gulf of Mexico. Fishery Bulletin 99(4):617-627. Patterson, W. F., J. C. Watterson, R. L. Shipp, and J. H. Cowan. 2001b. Movement of tagged red snapper in the northern Gulf of Mexico. Transactions of the American Fisheries Society 130(4):533-545. Patterson, W. F., and J. H. Cowan, Jr. 2003. Site fidelity and dispersion of red snapper associated with artificial reefs in the northern Gulf of Mexico. Pages 181-193 in D. R. Stanley and A Scarborough-Bull, editors. Fisheries, reefs, and offshore development. American Fisheries Society Symposium 36. Bethesda, Maryland. Peabody, M. B. 2004. The fidelity of red snapper (Lutjanus campechanus) to petroleum platforms and artificial reefs in the northern Gulf of Mexico. Master’s thesis. Louisiana State University, Baton Rouge, Louisiana, 82 pp. Powers, S. P., J. H. Grabowski, C. H. Peterson, W. J. Lindberg. 2003. Estimating enhancement of fish production by offshore artificial reefs: uncertainty exhibited by divergent scenarios. Marine Ecology Progress Series 264:265277. 40 Rummer, J. L. 2007. Factors affecting catch and release (CAR) mortality in fish: insight into CAR mortality in red snapper and the influence of catastrophic decompression. Pages 123-144 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60.Bethesda, Maryland. Saillant, E., and J. R. Gold. 2006. Population structure and variance effective size of red snapper (Lutjanus campechanus) in the northern Gulf of Mexico. Fishery Bulletin 104:136-148. SAS Institute Inc., 2008. SAS Online Doc® 9.2. Cary, North Carolina. SAS Institute Inc. SEDAR (Southeast Data, Assessment and Review) 2005. Stock assessment report of SEDAR 7: Gulf of Mexico red snapper. Section 3. Assessment workshop. South Atlantic Fishery Management Council, Charleston, South Carolina. Available: www.sefsc.noaa.gov/sedar/(March 2007). Sherman, R. L., D. S. Gilliam, and R. E. Spieler. 2002. Artificial reef design: void space, complexity, and attractants. ICES Journal of Marine Science 59:S196S200. Strelcheck, A. 2001. The influence of reef design and nearest-neighbor dynamics on artificial reef fish assemblages. Master’s thesis. University of South Alabama, Mobile, Alabama, 137 pp. Strelcheck, A. J., J. H. Cowan Jr., and A. Shah. 2005. Influence of reef location on artificial-reef assemblages in the northcentral Gulf of Mexico. Bulletin of Marine Science 77(3):425-440. Strelcheck, A J., J. H. Cowan, Jr., and W. F. Patterson, III. 2007. Site fidelity, movement, and growth of red snapper: implications for artificial reef management. Pages 147-162 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Szedlmayer, S. T., and R. L. Shipp. 1994. Movement and growth of red snapper, Lutjanus campechanus, from an artificial reef area in the northeast Gulf of Mexico. Bulletin of Marine Science 55:887-896. Szedlmayer S. T. and J. D. Lee. 2004. Diet shifts of juvenile red snapper (Lutjanus campechanus) with changes in habitat and fish size. Fishery Bulletin 102(2):366-375. 41 Szedlmayer, S. T., and R. L. Schroepfer. 2005. Long-term residence of red snapper on artificial reefs in the northeastern Gulf of Mexico. Transactions of the American Fisheries Society 134:315-325. Watterson, J. C. 1998. Estimates of site fidelity and short term movements of red snapper (Lutjanus campechanus) based upon mark/recapture on north central Gulf of Mexico artificial reefs. Master’s thesis. University of South Alabama, Mobile, Alabama, 76 pp. Wells, R. J. D. 2007. The effects of trawling and habitat use on red snapper and the associated community. Doctoral dissertation. Louisiana State University, Baton Rouge, Louisiana, 179 pp. Wells, R. J. D., and J. H. Cowan, Jr. 2007. Video estimates of red snapper and associated fish assemblages on sand, shell, and natural reef habitats in the north-central Gulf of Mexico. Pages 39-57 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Westmeyer, M. P., C. A. Wilson, III, and D. L. Nieland. 2007. Fidelity of red snapper to petroleum platforms in the northern Gulf of Mexico. Pages 105121 in W. F. Patterson, III, J. H. Cowan, Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society Symposium 60. Bethesda, Maryland. Wilson, C. A. and D. L. Nieland. 2001. Age and growth or red snapper, Lutjanus campechanus, from the northern Gulf of Mexico off Louisiana. Fishery Bulletin 99(4): 653-664). Workman, I., A. Shah, D. Foster, and B. Hataway. 2002. Habitat preferences and site fidelity of juvenile red snapper (Lutjanus campechanus). ICES Journal of Marine Science 59:S43-S50. 42 Tables 43 Table 1. Latitudinal and longitudinal coordinates for the sections of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Northwest Corner Northeast Corner Northeast Corner2 Southwest Corner Southeast Corner A 30 04.000N 88 32.400W 30 04.000N 88 31.700W 30 01.700N 88 29.300W 30 01.374N 88 32.406W 30 01.374N 88 29.300W B 30 01.374N 88 32.406W 30 01.374N 88 29.300W 30 00.240N 88 32.406W 30 00.240N 88 29.300W C 30 00.240N 88 32.406W 30 00.240N 88 29.300W 29 59.200N 88 32.400W 29 59.200N 88 29.300W Section 44 Table 2. Latitudinal and longitudinal coordinates for each artificial reef pattern and total visits to each section and artificial reef pattern in artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Section Latitude Longitude Depth (m) Total Pattern Visitis A Clump OL100 OL200 OL300 30 03.734N 30 03.770N 30 02.242N 30 01.960N 88 32.339W 88 31.506W 88 30.120W 88 30.645W 21.3 20.4 24.0 24.0 8 5 4 6 B Clump OL100 OL200 OL300 30 00.760N 30 01.141N 30 00.697N 30 01.129N 88 31.591W 88 29.531W 88 29.544W 88 31.009W 25.0 25.3 25.3 25.0 8 9 7 9 C Clump OL100 OL200 OL300 30 00.178N 30 00.013N 29 59.833N 29 59.713N 88 30.471W 88 32.009W 88 31.594W 88 31.154W 26.0 26.0 26.2 26.8 6 6 5 4 45 Pattern Table 3. Number of total visits to each pattern and total number of pattern visits by season at artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Season Clump Pattern OL100 Spring 5 6 5 6 Summer 11 11 7 7 Fall 6 3 5 6 Total 22 20 17 19 46 OL200 OL300 Table 4. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section A of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Location Date Traps Set Total Red Snapper CPUE (red snapper/trap soak hour) A Clump 10/26/2007 3/12/2008 4/22/2008 7/2/2008 7/16/2008 8/7/2008 10/3/2008 11/5/2008 3* 4 3** 3** 4 4 3** 4 7 5 12 43 19 2 10 18 1.17 0.63 2.00 7.17 2.38 0.25 1.67 2.25 3* 4 4 4 4 6 7 16 13 29 1.00 0.88 2.00 1.63 3.63 3** 4 4 4 11 3 31 16 1.83 0.38 3.88 2.00 4 4 3*** 4 4 4 7 33 29 18 11 14 0.88 4.13 4.83 2.25 1.38 1.75 N=8 A OL100 10/26/2007 3/12/2008 4/22/2008 7/2/2008 7/16/2008 N=5 A OL200 7/2/2008 8/7/2008 10/3/2008 11/5/2008 N=4 A OL300 3/12/2008 4/22/2008 7/16/2008 8/7/2008 10/3/2008 11/5/2008 N=6 *Only 3 traps were used per reef pattern on particular sampling trip. **Trap broken on pyramid upon retrieval. ***Rope became unattached from buoy upon retrieval, trap lost. 47 Table 5. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section B of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Location Date Traps Set Total Red Snapper CPUE (red snapper/trap soak hour) B Clump 9/28/2007 3/6/2008 4/30/2008 6/3/2008 6/10/2008 7/10/2008 7/17/2008 9/18/2008 4 4 4 4 4 4 4 4 2 6 10 8 1 16 7 10 0.25 0.75 1.25 1.02 0.13 2.01 0.88 1.25 4 4 4 4 4 4 4 4 4 4 0 19 2 23 25 16 16 17 0.50 0.00 2.38 0.25 2.88 3.13 2.00 2.00 2.13 4 4 4 4 4 4 4 10 10 6 1 22 10 18 1.25 1.25 0.75 0.13 2.75 1.25 2.25 4 4 4 4 4 4 3* 4 4 11 3 5 13 4 10 8 4 2 1.38 0.38 0.63 1.63 0.50 1.25 1.33 0.50 0.25 N=8 B OL100 9/28/2007 3/6/2008 4/2/2008 6/3/2008 6/10/2008 7/10/2008 7/17/2008 8/21/2008 11/20/2008 N=9 B OL200 3/6/2008 4/2/2008 4/30/2008 7/10/2008 8/21/2008 9/18/2008 11/20/2008 N=7 B OL300 9/28/2007 4/2/2008 4/30/2008 6/3/2008 6/10/2008 7/17/2008 8/21/2008 9/18/2008 11/20/2008 N=9 *Broken Trap 48 Table 6. Catch per unit effort (CPUE, red snapper/trap soak hour) of red snapper for each trip to individual artificial reef patterns in Section C of artificial reef site FH-13 located offshore of Mississippi in the Gulf of Mexico and sampled during the period of September 2007 through November 2008. Location Date Traps Set Total Red Snapper CPUE (red snapper/trap soak hour) C Clump 5/28/2008 6/16/2008 6/19/2008 6/24/2008 7/8/2008 11/6/2008 4 4 3* 4 4 4 3 23 9 26 19 3 0.38 2.88 1.50 3.25 2.38 0.38 4 4 4 4 4 4 4 9 19 6 24 7 0.50 1.13 2.38 0.75 3.00 0.88 4 4 4 3* 4 13 15 17 18 14 1.63 1.88 2.13 3.00 1.75 4 4 4 4 9 3 7 0 1.13 0.38 0.88 0.00 N=6 C OL100 5/28/2008 5/30/2008 6/16/2008 6/19/2008 6/24/2008 7/8/2008 N=6 C OL200 5/30/2008 6/19/2008 6/24/2008 7/8/2008 11/6/2008 N=5 C OL300 5/28/2008 5/30/2008 6/16/2008 11/6/2008 N=4 *Broken trap 49 Table 7. Recapture data for red snapper captured with trap nets during sampling from September 2007 through November 2008 at artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Capture Capture Recapture Recapture Days at Total Length Growth Date Site Date Site Large Increase (mm) (mm/day) 3/12/2008 A Clump 4/22/2008 A Clump 41 10 0.24 4/2/2008 B OL300 6/3/2008 B OL300 62 3 0.05 9/28/2007 B OL100 6/10/2008 B OL100 256 51 0.20 6/16/2008 C OL100 6/24/2008 C OL100 8 1 0.13 6/16/2008 C OL100 6/24/2008 C OL100 8 1 0.13 6/16/2008 C OL100 6/24/2008 C OL100 8 1 0.13 5/30/2008 C OL100 6/24/2008 C OL100 25 7 0.28 5/30/2008 C OL200 6/24/2008 C OL200 25 8 0.32 6/19/2008 C OL200 6/24/2008 C OL200 5 6 1.20 6/19/2008 C Clump 6/24/2008 C Clump 5 2 0.40 6/24/2008 C OL200 7/8/2008 C OL200 14 3 0.21 6/24/2008 C OL200 7/8/2008 C OL200 14 7 0.50 6/19/2008 C OL200 7/8/2008 C OL200 19 8 0.42 6/19/2008 5/30/2008* C OL200 7/8/2008 C OL200 19 7 0.37 C OL200 7/8/2008 C OL200 39 8 0.21 6/24/2008 C Clump 7/8/2008 C Clump 14 5 0.33 6/24/2008 C Clump 7/8/2008 C Clump 14 no growth 0.00 6/19/2008 C Clump 7/8/2008 C Clump 19 1 0.05 6/16/2008 C Clump 7/8/2008 C Clump 22 6 0.27 6/16/2008 C Clump 7/8/2008 C Clump 22 5 0.23 6/3/2008 B Clump 7/10/2008 B Clump 37 13 0.35 7/2/2008 A Clump 7/16/2008 A Clump 14 no growth 0.00 7/2/2008 A Clump 7/16/2008 A Clump 14 5 0.36 6/3/2008 B Clump 7/17/2008 B Clump 44 12 0.27 7/10/2008 B OL100 8/21/2008 B OL100 42 3 0.07 7/10/2008 B OL100 8/21/2008 B OL100 42 2 0.05 7/10/2008 B OL100 8/21/2008 B OL100 42 10 0.24 7/10/2008 B OL100 8/21/2008 B OL200 42 17 0.40 7/2/2008 A OL200 10/3/2008 A OL200 93 39 0.42 8/7/2008 10/3/2008* A OL200 10/3/2008 A OL200 57 32 0.56 A OL200 11/5/2208 A OL200 33 17 0.52 N=31 *Double recapture 50 Table 8. Condition and location of capture of red snapper which were released in any condition other than the best possible (Good) after being captured with trap nets from September 2007 through November 2008 at artificial reef site FH13 off the coast of Mississippi in the Gulf of Mexico. Location of Capture Date Captured Tagged Condition A OL200 A OL300 A OL300 A OL300 B OL100 B OL100 B Clump C OL200 A OL100 C OL100 C OL100 C Clump C OL100 B OL100 B OL300 C OL200 A OL300 A OL300 A OL300 A OL300 A OL300 A OL300 A OL300 A OL300 B OL100 B OL100 B OL200 B OL200 B OL300 8/7/2008 8/7/2008 7/16/2008 7/16/2008 7/10/2008 7/10/2008 7/10/2008 7/8/2008 7/2/2008 6/24/2008 6/24/2008 6/24/2008 6/19/2008 6/10/2008 6/3/2008 5/30/2008 4/22/2008 4/22/2008 4/22/2008 4/22/2008 4/22/2008 4/22/2008 4/22/2008 4/22/2008 4/2/2008 4/2/2008 3/6/2008 3/6/2008 9/28/2007 Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes No No No No No No Yes Yes No Yes Yes Fair Fair Fair Fair Fair Fair Fair Fair Fair Dead Dead Fair Fair Fair Fair Fair Fair Fair Dead Fair Fair Fair Fair Fair Dead Dead Fair Fair Fair N=29 51 Figures 52 53 Figure 1. Location of artificial reef site FH-13 in the northern Gulf of Mexico a. b. Figure 2. Pyramid structures used to construct artificial reef complexes at artificial reef site FH-13 in the northern Gulf of Mexico (2a) and a trap that was used for collecting fish during sampling (2b) (Photographs provided by the Mississippi Department of Marine Resources). 54 X XXX X Clump 100 ft XX X XXX X 100 ft XX Outlier 100 (OL100) 200 ft XX X XXX X 200 ft XX Outlier 200 (OL200) 300 ft XX X XXX X 300 ft XX Outlier 300 (OL300) Figure 3. Artificial reef patterns deployed within each section of artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. 55 2.5 Mean CPUE (Red snapper/h) 2 1.5 1 0.5 0 Clump OL100 OL200 OL300 Figure 4. Mean catch per unit of effort (CPUE; red snapper/trap soak-hour) by pattern with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids. 56 2.5 Mean CPUE (Red snapper/h) 2 1.5 1 0.5 0 Spring Summer Fall Figure 5. Mean catch per unit of effort (CPUE; red snapper/trap soak-hour) by season with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November). 57 300 Mean total length (mm) 250 200 150 100 50 0 Clump OL100 OL200 OL300 Figure 6. Mean total length (mm) by pattern with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids. 58 300 Mean total length (mm) 250 200 150 100 50 0 Spring Summer Fall Figure 7. Mean total length (mm) by season with associated standard error bars for red snapper captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November). 59 Clump Total Number Red Snapper Total Number Red Snapper 80 70 60 50 40 30 20 10 0 80 70 60 50 40 30 20 10 0 Length Group (mm) OL200 Length Group (mm) Total Number Red Snapper 60 Total Number Red Snapper Length Group (mm) 80 70 60 50 40 30 20 10 0 OL100 80 70 60 50 40 30 20 10 0 OL300 Length Group (mm) Figure 8. Red snapper length frequency distributions by reef pattern type. Length measurements are total lengths (mm). Red snapper were captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. The clump pattern consists of five closely spaced pyramid structures, and the outlier patterns consist of five closely spaced pyramids and two sets of two outlier pyramids at 100 ft (OL100), 200 ft (OL200), and 300 ft (OL300) from the main clump of pyramids. Total Number Red Snapper 180 160 140 120 100 80 60 40 20 0 Spring Total Number Red Snapper Length Group (mm) 180 160 140 120 100 80 60 40 20 0 Summer Total Number Red Snapper Length Group (mm) 180 160 140 120 100 80 60 40 20 0 Fall Length Group (mm) Figure 9. Red snapper length frequency distributions by season. Length measurements are total lengths (mm). Red snapper were captured with trap nets during September 2007 through November 2008 from artificial reef site FH-13 off the coast of Mississippi in the Gulf of Mexico. Seasons in which sampling took place were spring (March, April, and May), summer (June, July, and August) and fall (September, October, and November). 61 2.5 R² = 0.004 Loge CPUE (Red snapper/h) 2 1.5 1 0.5 0 Loge CPUE (Red snapper/h) 1 1.5 2 2.5 Square Root Dissolved Oxygen (mg/L) 2.5 3 R² = 0.006 2 1.5 1 0.5 0 31 32 33 34 Salinity (ppt) 35 Loge CPUE (Red snapper/h) 2.5 36 R² = 0.027 2 1.5 1 0.5 0 15 17 19 21 23 Temperature (Celsius) 25 27 29 Figure 10. Relationship between red snapper catch per unit of effort (CPUE; red snapper/trap soak-hour) and environmental variables (dissolved oxygen, salinity, and temperature) in the Mississippi artificial reef site FH-13, Gulf of Mexico, from September 2007 through November 2008. 62 APPENDIX A TOTAL NUMBER OF FISH BY SPECIES COLLECTED WITH TRAP NETS FROM SEPTEMBER 2007 THROUGH NOVEMBER 2008 AT EACH ARTIFICIAL REEF PATTERN WITHIN EACH SECTION OF ARTIFICIAL REEF SITE FH-13 LOCATED OFFSHORE OF MISSISSIPPI IN THE GULF OF MEXICO. 63 A B C Species Clump OL100 OL200 OL300 Clump OL100 OL200 OL300 Clump OL100 OL200 OL300 Total % of Total Lutjanus campechanus 116 71 61 112 60 122 77 60 83 69 77 19 927 66% 12 5 0 0 7 10 6 6 14 14 4 3 81 6% 0 1 0 0 0 0 0 0 0 0 0 0 1 < 1% 6 7 2 2 0 5 0 1 0 2 2 0 27 2% 0 0 0 0 1 0 0 0 0 0 0 0 1 < 1% 1 0 2 0 0 0 0 0 0 0 0 0 3 < 1% 28 52 17 22 1 9 1 0 0 4 3 2 139 10% 5 8 0 1 2 12 1 1 5 4 1 1 41 3% 5 3 0 0 1 4 0 0 2 0 2 0 17 1% 4 6 0 3 5 8 2 5 8 3 5 2 51 4% Red snapper Lutjanus synagris Lane snapper Lutjanus griseus Gray snapper Mycteroperca microlepis Gag 64 Mycteroperca phenax Scamp Epinephelus nigritus Warsaw grouper Balistes capriscus Gray triggerfish Lagodon rhomboides Pinfish Haemulon aurolineatum Tomtate Centropritis philadelphica Rock sea bass Continued; A B C 65 Species Clump OL100 OL200 OL300 Clump OL100 OL200 OL300 Clump OL100 OL200 OL300 Total % of Total Micropogonias undulates Atlantic croaker 2 0 0 0 16 3 11 12 2 0 0 1 47 3% Equetus umbrosus Cubbyu 1 0 0 0 4 0 0 0 1 1 0 1 8 < 1% Orthopristis chrysoptera Pigfish 14 12 0 4 0 3 0 1 0 2 1 0 37 3% Rypticus maculates Whitespotted soapfish 2 0 0 0 1 0 1 3 0 0 1 0 8 < 1% 0 0 0 0 1 1 0 0 0 0 0 0 2 < 1% Carcharhinus limbatus Blacktip shark 0 0 0 0 1 0 0 0 0 0 0 0 1 < 1% Cynoscion arenarius White trout 0 0 0 0 1 0 0 0 0 0 0 0 1 < 1% Menticirrhus americanus Southern kingfish 0 0 0 0 0 1 0 0 0 0 0 0 1 < 1% Arius felis Hardhead catfish 0 0 0 0 1 0 2 2 0 0 0 0 5 < 1% Bagre marinus Gafftopsail catfish 0 0 0 0 1 0 0 0 0 0 0 0 1 < 1% Opsanus pardus Leopard toadfish 0 0 0 0 0 1 0 1 1 0 0 0 3 < 1% Total Number of Fish 196 165 82 144 103 179 101 92 116 99 96 29 1402 Rhizoprionodon terraenovae Atlantic sharpnose shark