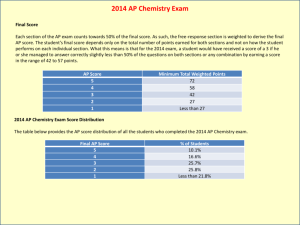
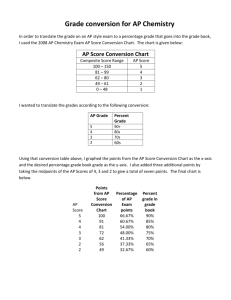
2010 * 2011 - Grapevine-Colleyville Independent School District
advertisement

2010 – 2011 ADVANCED PLACEMENT CHEMISTRY Instructor: Ponzell Goff E-mail: ponzell.goff@gcisd.net Class Website: Grapevine-Colleyville ISD website, click on my name and open the AP Chemistry Lessons Page Welcome to AP Chemistry! Like a first-year chemistry course, AP Chemistry explores the behavior and properties of matter. Extending upon topics presented in first-year chemistry, the AP Chemistry course is designed to answer these and many other questions: • How does observed behavior support current atomic theory? • How are patterns of bonding related to the behavior of substances? • What changes occur during nuclear reactions • How can the macroscopic behavior of gases, liquids and solids and solutions be explained at the molecular level. • What factors influence the stability of a substance? • What factors are involved in whether or not a reaction will occur? To what extent will reactions occur? Why will some reactions not occur or occur only slowly? • How are energy changes related to the nature of chemical reactions and the observed behaviors of substances. • What laboratory techniques are employed to discover the answers to these and other questions? Ensuring Success in Chemistry If you have a hobby or are involved in organized sports, then you already know the key to being successful in the activity:practice. In the same way you ensure success in a sport, continued practice in a course ensures that you learn well and achieve at a high level. In order to become proficient – which is your goal – your must commit to being actively involved, practice regularly and keep up when new ideas are investigated or new content is introduced. It is expected that you have matured to a point where you understand the responsibility you have for your own learning. Taking an active role in your learning is necessary for successful completion of this or any other course. Active involvement in class also provides you with good practice in developing a lifelong pattern of setting high expectations for yourself and exploring ways to meet those expectations. I will encourage your development and provide you with methods of promoting success; but you must recognize that you are ultimately responsible for the quality of your achievement. Whether you're struggling with a specific topic or online activities and study, teacher designated before or after school tutoring and supplemental materials for review. Page instruction in order to ensure mastery of or learn more about the course content. Reinforcement opportunities include 1 you're interested in further examination of a topic, there will be plenty of opportunities for you to support classroom It is a common misconception that teachers assign grades for performance. It is, in fact, students that decide on the grade she or she receives; teachers simply perform the math on your scores. You will be given plenty of opportunities to demonstrate your assessment will be based on your ability to understand the material at the recall, application, performance and extension levels. Thus, you should focus your study to be able to: show that you have an understanding of basic show that you can apply the facts in a useful way show that you can perform activities and procedures essential to understanding chemistry show that you can explain relationships and observations in terms show that you can critically explore the material beyond what has been explicitly discussed show that you can develop hypotheses that can be tested and supported through the use of the scientific method and analyze laboratory data Assessment Program The philosophy of the assessment program is twofold: first, assessment is used to present to the student a fair and accurate measure of his or her performance based on previously established learning objectives; second, assessment is used to evaluate strengths and weaknesses of student habits and/or remedial study of previous material. In order to adequately perform each objective of assessment, a regular program of evaluation of student performance is necessary. Hence, the following testing program will be used for the lecture section of Advanced Placement Chemistry for 2010-2011. • Quizzes consisting of both multiple-choice and/or free-response items will be given through-out the unit. • One 90-minute Examination will be given per 6 weeks grading period. A students’ course grade for each 6 weeks grading periods are according to the following scale. • [Earned points/total points] x 100 = % grade COURSEWORK There are several types of coursework that you will complete during the course, which are shown here. • Examinations an AP-like scale. • Practice Sets Page the material discussed in the most recently completed assignment set. All Examinations are scored and recorded to 2 Exams will be cumulative in nature. The exams will cover material from the beginning of the course up to Practice sets from your textbook are included below. The sets constitute a minimum requirement for successful completion of the course. The due dates for the assignment will be set for the days that they will be reviewed in class. Of course, you are always free to ask about those items with which you are having trouble. You should expect an amount of practice that ensures appropriate practice of material to allow for your mastery of content. Only with adequate practice will you be prepared to complete the AP Chemistry exam with a score that will provide college credit. The assigned problems provide a minimum of practice that generally wellprepares students; your needs may be different. Use classroom test scores to evaluate y our need to complete additional unassigned problems and practice. Practice sets will be assigned at the beginning of each major unit of study, and it will be divided to provide recommendations for pacing. For questions about an assignment, answer or solution, there are several options available: • Ask in class - I see you every class period, and I enjoy talking with you. • View the solutions in the example problems provided in the curriculum textbook. • View the solutions provided in the Solution's Guide. • See me before school, during my planning period, or after school. • Quizzes In-class quizzes will be given frequently so that we may measure performance and success in a way that ensures objectives are being met and goals are being reached. Please be prepared for significant quizzing surrounding the assignment sets and laboratory activities. Assignment sets will be collected. Completion of all assignments for a particular Quiz will result in the “curving” of your Quiz to the AP scale; otherwise, your Quiz will be straight-scaled. • Free-Response Items Free-response practice items and assessment items will be given frequently in class. No graded free response item will be given prior to the completion of practice free-response items or assignment set practice of a concept. You may not receive advance notice of graded free-response items. Significant free-response practice should be expected prior to course examinations. • Laboratory assignments will contribute to your laboratory grade. Page concept of the current lab investigation or of general lab procedures relevant to a particular lab. The pre-lab 3 Laboratory Pre-Lab Assignments – Pre-lab assignments will generally surround your understanding of the Laboratory Notebook/Follow-Up – At the conclusion of each lab, there will be available online or on paper a series of follow-up questions or discussion questions to address. After completion, you should turn in raw data for the lab (in your lab notebook), which you will have formalized and analyzed in the lab follow-up activity. Laboratory Performance: Lab performance – including associated behavior and safety – will be assessed laboratory activities throughout the year. ABSENCES Absences are difficult from the point-of-view of teachers in that they require additional time be dedicated to material that was previously discussed, maintenance of records and sorting and organization of materials. Absences are difficult form the point-of-view of students in that they require making-up missed work and getting materials of instruction that were delivered during an absence. You are well-advised to minimize the number of days you are not present. • Make-up Class Assignments – Any assignments made while you are absent are due a number of days later equal to the number of days you missed. These assignments will receive a score of zero until you turn them in. This does not include assignments due on the day of your absence – these are due on the day you return. Except for tests and quizzes you missed on the day you are absent, I will not remind you about make-up work or that you have items to turn-in to me. Long-term assignments do not require this time extension. It is decreed that a long-term assignment is one of which you are aware one week before it is due. • Make-up Quizzes and Examinations – Make up quizzes and examinations must be completed within a timeframe established upon your return to school. This should generally be within a number of days equal to the number you were absent. This means if you miss a single day of school that you are expected to take the quiz or exam on the day you return. Make-up quizzes and exams cannot be completed during class. You must arrange to arrive before school, stay after school, take the quiz or test during lunch or otherwise find a block of time during which you can complete a make-up quiz or exam. No matter what time you choose, you need to arrange the make-up with me and not simply show up to take the quiz or exam; I would like to know at least the day before you plan on taking the quiz or exam. • No Make-up Labs and Demonstrations are given – The most difficult type of curricular activities to make up are laboratory investigations and viewing of classroom demonstrations. You are responsible for procedures, data and observations of all demonstrations and lab activities whether you have participated or not. LABORATORY INFORMATION • Lab Safety – Your study of chemistry will provide many opportunities for laboratory investigations. In a laboratory setting, your first priority is to maintain a safe environment for yourself, your classmates and other people in the building. The safety agreement outlines the behaviors expected, policies in effect and procedures to be followed in the laboratory setting by everyone in the lab. An accident here could cause serious injury or death. Therefore, a policy of zero-tolerance for dangerous activity within the lab is in effect. Prior to beginning Page each lab we perform. You are expected to behave at all times. 4 any lab work, you must complete the our Schools’ Laboratory Safety Quiz. We will discuss laboratory safety with • Lab Procedures – Separate packets will be given to you that discuss the expectations of behavior in the lab and detailing the procedures for performance of lab investigations. You are expected to have these with you at all times. You will have a lab folder in which you may place them. • Laboratory Work – An integral part of the course is the investigation of chemical processes through lab activities. Each year, we find ways to incorporate additional direct learning into the lab, and this year is no exception. We will make extensive use of our laboratory resources to introduce, reinforce and extend the topics explored in an AP Chemistry course. All laboratory work is to be recorded in a laboratory notebook. Additionally, you must return the Lab Safety Agreement, which is available online. This must be signed by you and a parent/guardian prior to beginning laboratory work. TEXTBOOKS You will be issued a textbook for the course that you use extensively so please bring to class each day. THE AP CHEMISTRY EXAM It is assumed that students have registered for this course in order to successfully complete the AP Chemistry exam in May. More information about this will be provided. Students should be aware that the expectations and requirements of the course are not varied for "takers" and "non-takers.” All expectations remain the same for all students during the April and May review period, as well. If you elect not take the College Board Exam, you will be required to take the District Exam. THE COURSE WEB SITE The course Web site offers many resources to supplement the in-class discussions and activities. Please visit the site regularly to complete online activities and to use the study guides . 2010 - 2011 ADVANCED PLACEMENT CHEMISTRY COURSE SYLLABUS Although circumstances may require slight adjustments to the schedule outlined below, the rigor of the course and inflexible deadlines dictate a relatively strict adherence to the syllabus. It should be understood that in an Advanced Placement course days on which schools experience unscheduled closings should be used for completion of upcoming assignments and/or remediation, as needed (that is, requirements for completion of assignments are not affected by a school closing). Assignment Set details begin on Page 14 Objectives: Page 5 Unit 1: Fundamental Review Identify the character, location and nature of the subatomic particles in the atom Describe the relationship between the subatomic particles and atomic number, mass number and ion charge Calculate average atomic mass from natural abundances Describe the formation of molecular compounds, ionic compounds and compare the properties of these classes of compounds Determine empirical and molecular formulas from percent composition data & reaction analysis data Calculate percent composition Determine the formulas and describe the nature of hydrated compounds Discuss the relationships given by chemical equations Use chemical equations to determine limiting reactants and product yields in chemical reactions Name compounds using the Stock System of naming Name acids, polyatomic ions, and hydrated compounds Predict valence electron structure from elements’ locations on the periodic table Assign and predict oxidation states to atoms, ions and compounds Describe the solution process for molecular and ionic compounds Describe and predict the electrolytic properties of solutions based upon the character of the solute Predict the solubility of ionic and molecular compounds, including acids and bases Write ionization/dissociation equations for appropriate compounds, and write full molecular, ionic and net ionic equations for reactions in aqueous solution Calculate solution concentration, and determine volumes of solutions required based upon concentration data Calculate required amounts for and perform dilutions Predict the occurrence of metathesis reactions and write their chemical equations using appropriate phase indicators Use the ideal gas equation and perform gas stoichiometry Among others, the following AP-recommended labs will be completed during this time: determination of a formula, determination of the percentage water in a hydrate, standardization of a solution using a primary standard, determination of mass-mol relationship in a chemical reaction, gravimetric analysis, determination of concentration by acid-base titration (strong acids and strong bases) Unit 2: The Periodic Table Objectives: Compare the relative sizes of the atoms of elements based upon their location on the periodic table and explain why & how the radii of atoms changes across a period and down a group Identify the property of ionization energy and discuss and explain the periodic trend of ionization energy; justify irregularities in the pattern of ionization energy irregularities in the pattern of electron affinity Describe the effects of atomic radius, ionization energy and electron affinity on the observed properties of 6 Identify the property of electron affinity and discuss and explain the periodic trend of electron affinity; justify Page atoms; e.g., formation of ions and reactivity Characterize elements as metals, nonmetals or semi-metals and discuss their behavior in relation to their metallic or nonmetallic character Unit 3: Electrochemistry I Objectives: Identify oxidation and reduction of chemical species; identify oxidants and reductants Describe the nature of a redox reaction Use the activity series of the metals to predict the outcome of a proposed redox reaction Balance redox reactions by the half-reaction ion method Characterize species as oxidizers or reducers, and predict the redox nature of species, including predicting the products of potential oxidations and reductions _Relate oxidation and reduction ability to the periodic table Describe the fundamentals of an electrochemical cell, and identify the essential characteristics of a cell Determine the cell potential for a given cell Determine reduction potentials for half-reactions used in cells Propose and construct spontaneous electrochemical cells Use shorthand notation for describing electrochemical cells Identify the nature of batteries and how their structure relates to their function Among others, the following AP-recommended labs will be completed during this time: determination of concentration by redox titration, determination of electrochemical series, measurements using electrochemical cells (measurement limited to voltage; otherwise, qualitative observations only). Unit 4: Chemical Kinetics Objectives: Explain the concept of reaction rate Describe, predict and discuss the factors that affect rates of reaction Explain reaction rates as they relate to the stoichiometry of chemical reactions Discuss, determine and use rate constants Calculate rate laws Determine concentrations based on rate integrated rate laws and half-life Interpret graphical data to draw conclusions about reaction orders Explain activation energy and transition states Use the Arrhenius equation and explain its significance; determine activation energy and rate constants at Recognize that reactions may occur in steps, and discuss the various steps of reactions Determine whether or not proposed mechanisms of reaction are consistent with observed reactions Define, identify and discuss the significance of a catalyst in a chemical reaction Page 7 variable temperatures Express nuclear decay via equations and apply them in terms of first-order chemical kinetics Among others, the following AP-recommended labs will be completed during this time: determination of the rate of reaction and its order, colorimetric or spectrophotometric analysis Unit 5: Chemical Equilibrium I Objectives: Describe the nature and behavior of systems at equilibrium Graphically represent equilibrium and establish the kinetics-equilibrium relationship Write equilibrium expressions Predict equilibrium behavior using the magnitude of Kc Justify and perform the manipulation of equilibrium constants Calculate equilibrium constants, and use equilibrium constants to determine equilibrium concentrations Determine and use the reaction quotient Predict the behavior of systems using Le Châtelier's Principle; propose changes to a system that will elicit desirable responses Among others, the following AP-recommended labs will be completed during this time: determination of the equilibrium constant for a chemical reaction, colorimetric or spectrophotometric analysis Unit 6: Chemical Equilibrium II: Acid/Base Equilibrim Objectives: Identify and characterize Arrhenius and Brønsted-Lowry acids and bases Identify and characterize conjugate acid-base pairs Predict the relative strengths of acids and bases Characterize the acid or base character relative to the concentrations of hydrogen ion and hydroxide ion Use the pH scale, measure pH and convert using relationships provided by Ka, Kb, Kw, and pOH; determine pH Identify and describe the character of strong acids and strong bases Identify and describe the character of weak acids and weak bases Write equilibrium expressions for weak acids and weak bases; determine the pH of weak acid and weak base solutions Determine equilibrium concentrations of ions and molecular species in acid-base equilibria Justify the relationship between percent ionization and molarity of an acid Predict and calculate the value of the acid-dissociation constants for polyprotic acids Predict the acid-base-neutral character of salt solutions; use Le Châtelier's Principle to justify predictions Predict acid-base strength and character based on chemical structure; make relationships to acid-base character using the periodic table Page 8 and observations Predict behavior of solutions exhibiting the common-ion effect Determine the pH of solutions exhibiting the common-ion effect Characterize and determine the pH of buffered solutions; use the Henderson-Hasselbalch equation Perform titrations, make predictions as to the character of solutions during titration, and determine pH of solutions during various points in a titration Interpret titration curves and justify discussions about significant regions of a titration curve Use and calculate the solubility-product constant; predict the occurrence of dissolution and precipitation using Ksp Predict how the nature of solutions affects solubility using the concepts of chemical equilibrium Among others, the following AP-recommended labs will be completed during this time: determination of concentration by acid-base titration (weak acids and weak bases), determination of appropriate indicators for various acid-base titrations; pH determination, synthesis of a coordination compound and its chemical analysis, preparation and properties of buffer solutions, separation and qualitative analysis of cations and anions Unit 7: Thermochemistry, Thermodynamics and Electrochemistry II Objectives: Identify and establish relationships between work, energy and heat Recognize kinetic energy and potential energy and its conversions, and discuss and describe thermal and internal energy Recognize and use the units of energy joule and calorie Identify and characterize a system and surroundings when discussing the transfer of energy; recognize and describe state functions; characterize endothermic and exothermic processes Characterize and apply the first law of thermodynamics to processes Define, apply and characterize enthalpy Justify and apply Hess’s Law; determine the enthalpies of formation of various compounds Discuss and perform calculations using calorimetry Characterize and predict spontaneity; relate spontaneity to equilibrium conditions Characterize, apply, calculate and predict entropy changes; establish and predict microscopic character from bulk observations Calculate free-energy changes; characterize the relationship between free energy and temperature, and predict spontaneity based on the free energy difference determined when evaluating the enthalpy/entropy relationship Quantitatively relate emf of a redox reaction to free energy change; use the Nernst equation to establish the Calculate changes in reactants/products during electrolysis and concentration cells. Quantitatively describe relationship between concentration and cell emf; relate the Nernst equation to Le Châtelier's Principle change associated with a chemical reaction, measurements using electrochemical cells and electroplating Page Among others, the following AP-recommended labs will be completed during this time: determination of enthalpy 9 oxidation-reduction reactions in terms of amperage, voltage and time. Unit 8: Principles of Chemical Bonding Objectives: Describe the nature of ionic bonding; predict the occurrence of ionic bonding; perform qualitative predictions of the lattice energy based on ionic size and ionic charge Describe the nature of covalent bonding; predict the occurrence of covalent bonding Use bond enthalpies to estimate reaction enthalpies; use bond enthalpies to discuss the relative strength of covalent bonds Describe and prepare Born-Haber cycles to estimate lattice energies Predict and characterize polar bonds based on electronegativity differences Draw Lewis structures and calculate formal charge; predict and justify exceptions to the “octet rule” Predict the occurrence of resonance, and justify the concept in terms of structural observations Determine the electronic and molecular geometries of molecules Use electronic and molecular geometries to explain substances’ behaviors Use the concept of hybrid orbitals to construct viable explanations of observed patterns of bonding Use electron configurations to explain observed molecular properties Unit 9: Solids, Liquids and Gases Objectives: Identify the intermolecular forces of attraction and repulsion that exist between molecules and ions in compounds and in mixtures Describe the relationships between the observed behavior of substances and mixtures and the intermolecular forces of attraction present in the substance or mixture; predict behavior based on intermolecular forces Discuss how size, composition and molecular shape affect the observed behavior of substances and mixtures Define and explain viscosity and surface tension; establish relationships between these physical properties and IMF Predict the occurrence and explain the behavior of hydrogen bonding Recognize the enthalpy changes that accompany phase changes, and interpret or produce a phase diagram Define and discuss vapor pressure Describe the behavior of solids based on their crystalline structure and molecular structure Characterize the solution process and predict the occurrence of dissolution Justify Henry’s law on the molecular level; perform calculations using Henry’s Law Express the concentration of solutions in various units Characterize the behavior of solutions versus pure solvents; explain the observed behaviors of solutions versus pure solvents; perform calculations to determine the behavior of solutions versus pure solvents Page 10 for a substance Provide justification for the kinetic molecular theory using observations of the behavior of matter Quantitatively describe the behavior of gases in terms of the gas laws (including Dalton’s law and Graham’s Law) Characterize and predict ideal and real behavior; derive and use the ideal gas equation; justify the use of the van der Waals equation and use to predict variations from ideal behavior Among others, the following AP-recommended labs will be completed during this time: determination of molar mass by vapor density, determination of the molar volume of a gas, determination of molar mass by freezing-point depression Unit 10: Review of Atomic Structure and the Bohr Model Objectives Discuss and characterize the experiments and chronology leading to the development of modern atomic theory Determine the wavelength, frequency and energy of electromagnetic radiation using the fundamental equations of light and energy Students will be able to describe the Bohr model of the atom, and use it to discuss the nature of spectral emissions Characterize the Quantum Mechanical Model of the atom Discuss an orbital in terms of its unique set of quantum numbers Assign the ground state configuration of atoms (including copper and chromium) and use ground state configurations to explain and predict observed behavior Justify observations related to unusual orbital-filling patterns In addition to these formal units of study, the following material is woven throughout the Advanced Placement Chemistry course: Reaction Chemistry, Concepts of Laboratory and Organic Chemistry, the remaining APrecommended labs. Unit 11: Applications of Chemistry in biology, the environment and industry by way of reviewing course concepts. Assignment Sets 2010 – 2011 – All assignments may not be listed below, as student achievement and interest likely require additions or deletions. Please see the course Web site for due dates and changes to this syllabus. The homepage of the course Web site will be the definitive source for due dates, assignments and other course information. Page Unit 1: Fundamental Review Coursework 11 Virtual Chem-Labs will be recommended or completed in-class where appropriate. Assignment Sets • Set A: 2.9a, 2.24, 2.25, .33a, 2.39, 2.42, 2.48, 2.50, 2.53, 2.54, 2.56, 2.58, 2.60, 2.61, 2.62, 2.68 (choose any Three) • Set B: 3.11 (as needed), 3.13 (choose any three), 3.17, 3.18, 3.23, 3.25 (choose any one), 3.29, 3.33, 3.35, 3.37, 3.41, 3.43, 3.47, 3.49, 3.51, 3.52, 3.53, 3.54, 3.57, 3.63, 3.71, 3.73, 3.77, 3.89, 3.98, 3.105 • Set C: 4.5, 4.16, 4.18, 4.49, 4.50, 4.60, 4.64, 4.66, 4.68 • Set D: 10.18, 10.29, 10.34, 10.38, 10.40, 10.53, 10.63, 10.68, 10.105a • Set D-2: 4.20, 4.24, 4.25, 4.27, 4.29, 4.30, 4.33, 4.38, 4.40, 4.43, 4.69, 4.79a, 4.79b, 4.81, 4.83, 4.85, 4.99, • Free-Response: The Unit 1 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Determining the Presence of Cations and Anions in Solution • Determination of a Formula: MgO Synthesis • Determination of Percentage Water in a Hydrate • Acid-Base Titration: Standardization and Percent Vitamin C in Commercial Tablet • Gravimetric Analysis/Determination of Mass-Mole Relationships • Synthesis of an Organic Compound Unit 2: Periodic Table Assignment Sets • Set E: 7.9, 7.13, 7.14, 7.21, 7.22a, 7.23, 7.26, 7.36a, 7.38a, 7.38b, 7.40, 7.41, 7.45, 7.53, 7.57, 7.63, 7.85, 7.91 (especially the difference between 4 and 5), 7.94 (second question), 7.96 • Free-Response: The Unit 2 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded. Unit 3:Redox /Electrochemistry 1 Assignment Sets • Set F: 4.47, 4.48, 4.50, 4.52, 4.53, 4.55, 4.56, 4.58, 4.95, 4.103, 20.5a, 20.11, 20.12, 20.13, 20.14, 20.19, 20.20 • Free-Response: The Unit 3 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Page 20.109a, 20.109c, 20.111, 20.113a, 20.113b 12 • Set G: 20.23, 20.29c, 20.31, 20.33, 20.35, 20.36, 20.37, 20.39, 20.41, 20.43, 20.45, 20.91, 20.93, 20.96, Lab Activities • Synthesis of a Coordination Compound and its Analysis by Redox Titration • Establishing a Table of Reduction Potential Unit 4: Kinetics Assignment Sets • Set H: 14.11, 14.13, 14.17, 14.21, 14.23, 14.25, 14.27, 14.31, 14.1, 14.3, 14.5, 14.7, 14.10 • Set I: 14.35, 14.37, 14.39, 14.41, 14.43, 14.45, 14.49, 14.51, 14.53, 14.55 • Set J: 14.14, 14.18, 14.33, 14.82, 14.84, 14.91, 14.94a - 14.94d, 14.104 • Set K: Arrhenius Equation and Mechanisms Practice Set • Free-Response: The Unit 4 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Colorimetric and Spectrophotometric Analysis • Determination of Rate of Reaction and Rate Order Unit 5: Chemical Equilibrium 1I Assignment Sets • Set L: 15.10, 15.13, 15.16, 15.17, 15.19, 15.21, 15.23, 15.24, 15.27, 15.30, 15.31, 15.33, 15.34 • Set M: 15.35, 15.37, 15.39, 15.43, 15.45, 15.47, 15.49, 15.51, 15.53, 15.57, 15.59, 15.62, 15.68, 15.71, 15.80 • Free-Response: The Unit 5 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities Page Unit 6: Acid-Base Equilibrium and Solubility Equilibrium 13 • Determination of an Equilibrium Constant Using Colorimetry Assignment Sets • Set N: 16.12, 16.13, 16.15, 16.16, 16.18, 16.19, 16.22, 16.23, 16.29, 16.31, 16.33, 16.35, 16.38, 16.39, 16.41, 16.43, 16.45, 16.46, 16.49 • Set O: 16.51, 16.53, 16.55, 16.57, 16.59, 16.61, 16.63, 16.66, 16.69, 16.73, 16.75, 16.77, 16.78 • Set P: 16.79, 16.81, 16.83, 16.87, 16.89, 16.91, 16.93, 16.95, 16.110 • Set Q: 17.9, 17.11, 17.15, 17.16, 17.17, 17.19, 17.21, 17.23, 17.25, 17.27, 17.29 • Set R: 17.31, 17.33, 17.34, 17.35, 17.36, 17.37, 17.38, 17.39, 17.41, 17.43, 17.44 • Set S: 17.45b, 17.47, 17.48, 17.49, 17.51, 17.53, 17.55, 17.56, 17.61, 17.63, 17.65, 17.69, 17.73 (choose one), 17.78, 17.87, 17.96, 16.117 • Free-Response: The Unit 6 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Hydrolysis of Salts/Determination of pH • Using Indicators in Acid-Base Chemistry • Separation and Analysis of Cations and Anions in Solution Unit 7: Thermochemistry / Thermodynamics Assignment Sets • Set A: 5.23, 5.25, 5.27, 5.33, 5.35, 5.36, 5.37, 5.40, 5.43, 5.44, 5.46, 5.60, 5.62, 5.64, 8.65, 8.69, 8.71, 8.72, 5.67, 5.70, 5.72 (choose two), 5.73, 5.87, 5.93, 5.98, 5.49, 5.50a, 5.52, 5.53, 5.57 • Set B: 19.8, 19.9, 19.12, 19.20a, 19.20b, 19.21, 19.25, 19.27, 19.29, 19.38, 19.39, 19.42, 19.48, 19.49, 19.51, 19.53, 19.55, 19.59, 19.63, 19.65, 19.69 (explain how [c] is obtained) • Set C: 19.73, 19.75, 19.79, 19.92a, 19.92b, 19.92d, 19.103, 20.58, 20.63, 20.80, 20.82, 20.85, 20.109 • Free-Response: The Unit 7 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Calorimetry: Determining the Energy Content of Foods/Determination of Enthalpy Change Associated with a Reaction • Quantitative Electrochemistry: Concentration Cells, Nernst Equation and Electrolysis (including Page Unit 8: Principles of Chemical Bonding 14 Electroplating) Assignment Sets • Set D: 7.29, 7.31, 7.34, 8.14, 8.16, 8.20, 8.23, 8.27, 8.38, 8.40, 8.43, 8.53 (consider only CO2 and CO), 8.97, 16.116 • Set E: 8.45, 8.48, 8.52, 8.55b, 8.61, 8.63, 8.86, 8.88 (choose two), 8.99, 8.101c, 8.101d (calculation only) • Set F: 9.14, 9.16, 9.19, 9.21, 9.23, 9.25, 9.27, 9.29, 9.35, 9.43, 9.47, 9.53, 9.57, 9.76, 9.96 • Free-Response: The Unit 8 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Molecular Modeling/Properties of Substances Unit 9: Solids, Liquids and Gases Assignment Sets • Set G: 11.5, 11.10, 11.13, 11.15, 11.17, 11.19, 11.21, 11.23, 11.24, 11.26, 11.32, 11.35, 11.37, 11.39, 11.45, 11.53, 11.55, 11.72, 11.79, 11.81a, 11.86, 11.101, 11.6 • Set H: 13.13, 13.15, 13.17, 13.21, 13.23 (second question), 13.27, 13.29, 13.35, 13.39, 13.41b, 13.41c, 13.41d, 13.45, 13.59, 13.64, 13.67, 13.95 • Set I: 10.21, 10.25b, 10.26, 10.30, 10.33, 10.37, 10.43, 10.47, 10.52, 10.56, 10.57, 10.59, 10.73, 10.75, 10.80, 10.83, 10.102, 10.112 • Free-Response: The Unit 9 free-response items will be distributed from time-to-time as we progress through the unit. Some will be collected and graded assignments. Lab Activities • Determination of Molar Mass by Vapor Density /Gas Constant / Molar Volume of a Gas • Determination of Molar Mass by Freezing-Point Depression • Determination of Molar Mass by Acid-Base Titration Units 10: Atomic Theory and Nuclear Chemistry Assignment Sets • Set J: A prepared set of items will be distributed for Atomic Theory. Particular attention will be paid to the Bohr Model of the atom and the applications of atomic spectra. the applications of nuclear chemistry to energy and medicine. Page to patterns of nuclear transmutations and nuclear stability, radioactive decay as a first-order process, and 15 • Set K: A prepared set of items will be distributed for Nuclear Chemistry. Particular attention will be paid Lab Activities • Applications of Atomic Spectra 2010 - 2011 Student Academic Honesty Statement for Submitted Work Student Last Name _________________________________ Student First Name _________________________________ By submitting work electronically or on paper for course credit constitutes my acknowledgement of and agreement to the following statement: I understand that the work I complete must be my own. If I submit work to you, then you may assume and I acknowledge that the submitted work is my own and was completed with only allowable resources. I also agree and you may assume that I did not discuss the assignment, including its contents, questions, or answers with other students unless explicit permission was given otherwise. I understand that the same obligation of integrity and honesty applies to electronically-submitted work as applies to any other work to which I have affixed my name. I understand that instances of academic dishonesty will be documented and the details will be forwarded to my administrator and the National Honor Society faculty advisor. Page 16 Student Signature: _________________________________________________________ Date: _______________, 2010