Chapter 24
advertisement

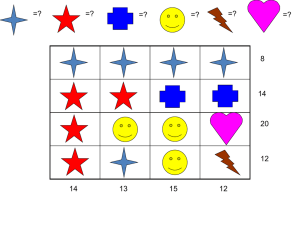
Chapter 24 Chemical Reactions In a chemical Reaction . . . Reactants Products • Substances reacting • New substances produced •Any state of matter •Follow law of conservation of mass Example: Which are the reactants? Which are the products? NaCl Na+ + Cl- 2HgO O2 + 2Hg NiCl2 + 2NaOHNi(OH)2 + 2NaCl Law of Conservation of Mass • In the late 1700s • Antoine Lavoisier • Mass is changed; NOT created or destroyed Example: 2HgO O2 + 2Hg 10.0g = 0.7g + 9.3g Try: Zn + 2HCl H2 + ZnCl2 12.0g 15.0g + 3.2g = 6.2g + _____ Chemical Changes in Equations • Table 1 on page 740 • We use formulas and equations to shorten the writing process • It helps us see how many atoms are involved Balancing Equations - Nuts and Bolts Lab Revisited 1. Build these compounds if Boltium (Bo) and Nutter (Nu) Bo2Nu3 BoNu Nu How many of each compound to balance Bo2Nu3 BoNu + Nu Balancing Equations - Nuts and Bolts Lab Revisited 2. Build these compounds BoNu2 Bo3 Nu How many of each compound to balance BoNu2 Bo3 + Nu Balancing Equations - Nuts and Bolts Lab Revisited 3. Build these compounds Bo2Nu2 Bo3 Nu How many of each compound to balance Bo3 + Nu Bo2Nu2 Balancing equations Example Steps 1. Write chemical 1. Lithium metal treated with equation with balanced water, a hydrogen gas and a molecules (cheat sheet lithium hydroxide are produced: for poly + diatomic) Li (s) + H2O LiOH + H2 (g) 2. Check equation for 2. Li 1 = 1 atom balance H 2 = 1 + 2 O 1 = 1 Balancing equations Steps 3. Choose coefficients to balance it 4. Recheck number of atoms Example 3. Li (s) + H2O LiOH + H2 (g) 4Li (s) + 4H2O 4LiOH + 2H2 (g) 4. Li 4 = 4 H 8 = 4 + 4 O 4 = 4