Slide 1
advertisement

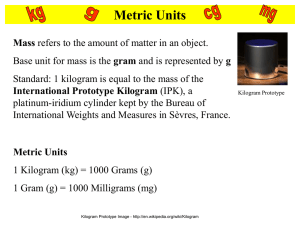
Chapter 1 Measurement and Problem Solving Units of Chapter 1 Why and How We Measure SI Units of Length, Mass, and Time More about the Metric System Unit Analysis Unit Conversions Significant Figures Problem Solving 1.1 Why and How We Measure Physics attempts to describe nature in an objective way through measurement. Measurements are expressed in units; officially accepted units are called standard units. Major systems of units: 1. Metric 2. British (used by the U.S., but no longer by the British!) 1.2 SI Units of Length, Mass, and Time Length, mass, and time are fundamental quantities; combinations of them will form all the units we will use through Chapter 14. In this text, we will be using the SI system of units, which is based on the metric system. 1.2 SI Units of Length, Mass, and Time SI unit of length: the meter. The original definition is on the left, the modern one is on the right. 1.2 SI Units of Length, Mass, and Time SI unit of mass: the kilogram Originally, the kilogram was the mass of 0.10 m3 of water. Now, the standard kilogram is a platinumiridium cylinder kept at the French Bureau of Weights and Measures. 1.2 SI Units of Length, Mass, and Time SI unit of time: the second The second is defined as a certain number of oscillations of the cesium-133 atom. 1.2 SI Units of Length, Mass, and Time In addition to length, mass, and time, base units in the SI system include electric current, temperature, amount of substance, and luminous intensity. These seven units are believed to be all that are necessary to describe all phenomena in nature. 1.3 More about the Metric System The British system of units is used in the U.S., with the basic units being the foot, the pound (force, not mass), and the second. However, the SI system is virtually ubiquitous outside the U.S., and it makes sense to become familiar with it. 1.3 More about the Metric System In the metric system, units of the same type of quantity (length or time, for example) differ from each other by factors of 10. Here are some common prefixes: 1.3 More about the Metric System The basic unit of volume in the SI system is the cubic meter. However, this is rather large for everyday use, so the volume of a cube 0.1 m on a side is called a liter. Recall the original definition of a kilogram; one kilogram of water has a volume of one liter. 1.4 Unit Analysis A powerful way to check your calculations is to use unit analysis. Not only must the numerical values on both sides of an equation be equal, the units must be equal as well. 1.4 Unit Analysis Units may be manipulated algebraically just as other quantities are. Example: Therefore, this equation is dimensionally correct. 1.5 Unit Conversions A conversion factor simply lets you express a quantity in terms of other units without changing its physical value or size. The fraction in blue is the conversion factor; its numerical value is 1. 1.6 Significant Figures Calculations may contain two types of numbers: exact numbers and measured numbers. Exact numbers are part of a definition, such as the 2 in d = 2r. Measured numbers are just that—for example, we might measure the radius of a circle to be 10.3 cm, but that measurement is not exact. 1.6 Significant Figures When dealing with measured numbers, it is useful to consider the number of significant figures. The significant figures in any measurement are the digits that are known with certainty, plus one digit that is uncertain. It is easy to create answers that have many digits that are not significant using a calculator. For example, 1/3 on a calculator shows as 0.33333333333. But if we’ve just cut a pie in three pieces, how well do we really know that each one is 1/3 of the whole? 1.6 Significant Figures Significant figures in calculations: 1. When multiplying and dividing quantities, leave as many significant figures in the answer as there are in the quantity with the least number of significant figures. 2. When adding or subtracting quantities, leave the same number of decimal places (rounded) in the answer as there are in the quantity with the least number of decimal places. 1.7 Problem Solving The flowchart at left outlines a useful problem-solving strategy. It can be used for most types of physics problems. 1.7 Problem Solving The table at left describes several types of examples that are used in the text. Review of Chapter 1 SI units of length, mass, and time: meter, kilogram, second Liter: 1000 cm3; one liter of water has a mass of 1 kg Unit analysis may be used to verify answers to problems Significant figures – digits known with certainty, plus one Review of Chapter 1 Problem-solving procedure: 1. Read the problem carefully and analyze it. 2. Where appropriate, draw a diagram. 3. Write down the given data and what is to be found. (Make unit conversions if necessary.) 4. Determine which principle(s) are applicable. 5. Perform calculations with given data. 6. Consider if the results are reasonable.