Laboratory and Chemical Safety
advertisement

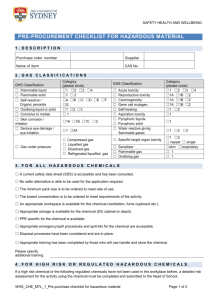
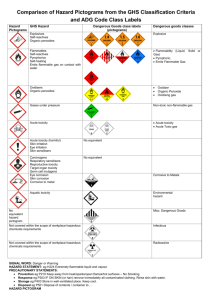
Laboratory and Chemical Safety • Part Two First Aid – Accidents • WATER – the first line for chemical contact or burns. Do not neutralize the chemical. The reaction will release heat and cause further damage to the skin • Cuts and other injuries. First aid can be applied by the instructor or the student can be referred to the campus health center. • If necessary, you will be taken to the school nurse, the doctor, or the emergency room. Please keep your insurance information with you at lab. Chemical Safety • Labeling – required to communicate chemical hazard to the person using it. • SDS – provides information about the chemical What is on the SDS • • • • • • • • • 0- Date of preparation 1- Identification (Formula and molecular weight) 2- Hazard identification 3- Composition / information on ingredients 4- First aid measures 5- Fire-fighting measures 6- Accidental release measures 7- Handling and Storage 8-Exposure control / personal protection • • • • • • • • 9- Physical and Chemical Properties 10- Stability and Reactivity 11- Toxocological information 12- Ecological information 13- Disposal considerations 14- Transport information 15- Regulatory information 16- Other information Where can you find an SDS • SDSs must be available in the lab in some form in case a student is injured. If the injury involves a chemical, we must have the SDS for the rescue squad. SDSs in the lab Yellow folder in wire cage – one page On a shelf in one of the two lab rooms - complete Other locations -In the CSO’s office MCK 307, Dr Crockett -On line (S:\Academic\CHEM\Chemical Inventory) GHS statements – 1 highest hazard, 5 lowest listed Acute toxicity – oral Category 1 - LD50 ≤ 5 mg/kg Category 2 - LD50 > 5 or ≤ 50 mg/kg Category 3 - LD50 > 50 or ≤ 300 mg/kg Category 4 - LD50 > 300 or ≤ 2000 mg/kg Acute toxicity – dermal Category 1 - LD50 ≤ 50 mg/kg Category 2 - LD50 > 50 or ≤ 200 mg/kg Category 3 - LD50 > 200 or ≤ 1000 mg/kg Category 4 - LD50 > 1000 or ≤ 2000 mg/kg Acute toxicity – inhalation Category 1 - LD50 ≤ 100 mg/kg Category 2 - LD50 > 100 or ≤ 500 mg/kg Category 3 - LD50 > 500 or ≤ 2500 mg/kg Category 4 - LD50 > 2500 or ≤ 5000 mg/kg Skin corrosion or Skin irritation (visible damage) Category 1A – Exposure < 3 min, Observation < 1 hour Category 1B – Exposure < 1 hour, Observation < 14 days Category 1C – Exposure < 4 hours, Observation < 14 days Category 2 – reversible adverse effects, Draize test: ≥ 2.3 < 4 Category 3 – reversible adverse effects, Draize: ≥ 1.5 < 2.3 Serious eye damage Category 1A – positive evidence from epidemiological studies Category 1B – positive evidence in in vivo germ cell tests, human germ cell test, or in vivo somatic mutagenicity tests Category 2 - suspected or possible tests, Reproductive toxicity Category 1A – known or presumed to cause effects on human reproduction or development based on human evidence Category 1B – known or presumed to cause effects on human reproduction or development based on experimental animals Category 2 – Human or animal evidence possibly with other information Category additional – concern for breastfed children effects on or via lactation Specific organ toxicity – single exposure Category 1 – significant toxicity to humans – based on reliable, good quality case studies or presumed toxic based on animal tests at low exposure Category 2 – presumed to be harmful to humans – based on animal tests at moderate exposure Category 3 – transient effects – narcotic effects or respiratory tract irritation Specific organ toxicity – repeated exposure Category 1 – significant toxicity to humans – based on reliable, good quality case studies or presumed toxic based on animal tests at low exposure Category 2 – presumed to be harmful to humans – based on animal tests at moderate exposure Eye damage / irritation Category 1 – Serious eye damage – irreversible damage 21 days after exposure Category 2A – eye irritation – reversible in 21 days Category 2B – mild irritant – reversible in 7 days Flammable liquids Category 1 – FP < 23 oC , initial BP ≤35 oC Category 2 – FP < 23 oC, initial BP > 35 oC Category 3 – FP ≥ 23 oC and ≤ 60 oC Category 4 – FP ≥ 60 oC and ≤ 93 oC Skin sensitization Germ cell mutagenicity Category 1A – Know or presumed to produce heritable mutations in human germ cells based on positive evidence from epidemiological studies Category 1B – Know or presumed to produce heritable mutations in human germ cells based on positive in vivo tests in mammals or human germ cells Category 2 – Suspected or possible. May include heritable mutations in human germ cells, positive evidence from animal tests Oxidizing solids – 3 categories Acute aquatic toxicity Category 1 – ≤ 1.00 mg/L Category 2 – > 1.00 ≤ 10.0 mg/L Category 3 – > 10.0 < 100 mg/L Chronic aquatic toxicity Category 1 – ≤ 1.00 mg/L, lack of rapid degradability or bioconcentration factor (BCF) <500 Category 2 – > 1.00 ≤ 10.0 mg/L , lack of rapid degradability unless BCF < 500 Category 3 – > 10.0 < 100 mg/L, lack of rapid degradability unless BCF < 500 Category 4 – > 100 mg/L, lack of rapid degradability unless BCF < 500 Carcinogenicity Category 1A – Known human carcinogen based on human evidence Category 1B – Presumed human carcinogen based on demonstrated animal carcinogenicity Category Two - Suspected carcinogen – based on limited evidence on human or animal GHS pictograms (nine) • Acute toxicity (fatal or severe) • Skin corrosion / burns • Eye damage • Corrosive to metals GHS • • • • • • Flammables Pyrophorics Self heating Emits flammable gas Self reactives Organic peroxides GHS • Explosives • Self-reactives • Organic peroxides • Aquatic toxicity GHS • • • • • • Irritant – skin and eye Skin sensitizer Acute toxicity (harmful) Narcotic effects Respiratory tract irritant Hazardous to ozone layer GHS • Gases under pressure • Oxidizers GHS • • • • • • Carcinogen Mutagenicity Reproductive toxicity Respiratory Sensitizer Target organ toxicity Aspiration toxicity Chemical Labeling - NFPA • • • • Blue – Health Hazard Red – Flammability Yellow – Reactivity White – Special symbol NFPA Colors • Blue – 4 – short exposure can cause death – ie. HCN. • Blue – 2 – chronic exposure could cause possible injury – ie. ammonia gas. • Red – 4 – readily vaporize and burn – propane gas. • Red – 2 – must be heated before combustion can occur – fuel oil. NFPA • Yellow 4 – capable of detonation at normal temperatures – TNT • Yellow 2 – violent chemical changes at elevated T / P or react violently with water – Ca metal • White – ₩ – water reactive – Mg metal. OX – oxidizer – ammonium nitrate ACID - acid ALK - base COR – corrosive - radioactive Dept. of Transportation • Explosives – five • Gases subcategories DOT • Flammable liquids • Flammable solids DOT • Oxidizers • Poisons DOT • Radioactive • Corrosives Other Hazards Our labeling – Blue • Blue – Health hazard, • • • • • • • • toxic NFPA 2 oral LD50 < 1 g / kg DOT class 6 (Poisons) TSCA listed GHS Acute toxic – oral, dermal, or inhalation GHS Warning – narcotic effects GHS Aquatic toxicity Reds • Dark Red – Flammable. • NFPA 2 • Flash point between 100o and 40 oC • DOT 3 or 4 (Flammable liquids or Flammable solids) • GHS flammable category 4 or oxidizer • Light red • NFPA 3 • Flash point between 40o and 10 oC • DOT 3 or 4 (Flammable liquids or Flammable solids) • GHS flammable category 2 or 3 or oxidizer • Combination of the two • NFPA 4 • Flash point below 10 oC • DOT 3 or 4 (Flammable liquids or Flammable solids) • GHS flammable category 1 or oxidizer Yellow – Corrosive or an oxidizer. NFPA 2 DOT 5 or 8. GHS corrosive, warning, or oxidizer Green – carcinogenic, teratogenic, or mutagenic. IARC 2 or listed on MSDS. GHS Health hazard Black – an explosive or one that can form an explosive species. DOT 1 (explosives) Listed as a peroxide former GHS – explosives White – “no hazard for now” GHS – no symbols Color dots Blue – toxic, red - flammable Blue – toxic, red on red – extremely flammable Color dots Blue – toxic, yellow – corrosive Blue – toxic, carcinogenic large green - Example • 26 – 697 – BY Shelf 26 - inorganic oxidizers MSDS number 697 B – blue – toxic Y – yellow corrosive Compound is 30% hydrogen peroxide Example • 10 – 642 – BR(3)YGX Shelf 10 – nitro organics MSDS number 642 B – toxic, R(3) – very flammable, Y – corrosive, X – explosive, G – carcinogenic 2-nitropropane (no longer on inventory) Chemical incompatibility • Alphabetical storage is not used completely – Sodium cyanide with sulfuric acid?? – Acids with bases? • Storage – functional groups. Organics from inorganics. Acids from alcohols. • We combine the two methods. – Separate the chemicals by functional group. – Alphabetize within the group. Waste from the labs • Labeled bottles will be in the hoods. • Be sure to place chemicals in the proper containers. Read the label! • Do not overfill a waste bottle (2/3 to 3/4). • If full, ask the stockroom manager for a new bottle. Do not use a beaker as a waste “bottle” in the hood. Labeling for Waste Bottles • Laboratory_______________________________ • Experiment ______________________________ • Waste Contents __________________________ • __________________________ • __________________________ • __________________________ • __________________________ • __________________________ Fire safety • Where are the fire exits when an alarm sounds? Look next to the lab or classroom door. Always check the route in a new building / classroom. • What is the procedure for exiting the building? Read the directions the first time you are in the room. • What is the procedure for after you exit the building? Go outside away from the building and get together with your class. Do not return to the dorm or classroom until the professor tells you to do so. You will be instructed by the building supervisor, police, or fire personnel. • Never assume that it is a drill. Always leave the building Reporting incidents • How do we report an accident? • Use the reporting form available. Where are the forms – in the lab, in the stockroom, or in my office. • Reportable accidents – – – – – Hazardous chemical spills Bodily injury Falls Simple first-aid Other as determined by CSO Accident report form • • • • • • • • • • • Name______________________________ Date_____________ Where exposure occurred: _____________________________________________________________________ ____________________________________________________________________ Description of exposure/incident _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ Type/name of chemical involved _____________________________________________________________________ *please send copy of the MSDS sheet for product with this form. Lab Superviser’s signature____________________________ First Aid – Accidents • WATER – the first line for chemical contact or burns. Do not neutralize the chemical. The reaction will release heat and cause further damage to the skin • Cuts and other injuries. Minor first aid can be applied by the instructor or the student can be referred to the campus health center. • If necessary, you will be taken to the school nurse, the doctor, or the emergency room. Please keep your insurance information with you at lab. Housekeeping duties – during lab • Broken glass - do not place into the trash cans. If glass is found in the trash, and unless we know differently, we will hold all students in the previous lab responsible. • Trash – trash can – not in the sinks or in the broken glass box • Chemical spills and waste – labeled bottles will be placed in the hood. Be careful to place the waste in the correct bottle if more than one bottle is present. Never fill the waste bottle over 2/3 to 3/4 full. Housekeeping after lab • • • • • • • • Clean all glassware that you used Wipe down the benchtop (wet paper towel) Return your tray to the cabinet (organic lab) Return used glassware to the lab supply (general chem) Refill burets with deionized water Check the sinks for paper, glass, or plastic Put the chairs in the center opening of the table. Leave the area as clean or cleaner as you found it. Chemical Hygiene Plan • What is the CHP? This is a document that tells us how to handle hazardous chemicals on the BC campus. • Covers. Transportation, handling, labeling etc on the BC campus. • Any questions should come to Dr Crockett • This will be available on line by later this term. References • • • • • • • Safety in Academic Chemistry Laboratories, ACS Handbook of Chemical Health and Safety, ACS Prudent Practices in the Laboratory, NRC Hazardous Chemicals and the Right to Know, Harris/Harvey Improving Safety in the Chemical Laboratory, Young Safe Storage of Laboratory Chemicals, Pipitone Environmental Compliance Assistance Guide for Colleges and Universities, APPA/CSHEMA • Waste Disposal in Academic Institutions, Kaufman • CFR • Laboratory Safety for Chemistry Students, Robert Hill and David Finster Quiz! • You will be given a quiz covering the basic safety rules and regulations that we have covered here. When finished, you will sign and turn in the quiz. This marks your attendance in the second half of this seminar. • If both the quiz and the dress code are not turned in, you will NOT receive credit for the seminar and you will no longer be allowed to work in our labs.