Home | EAT Notes
advertisement

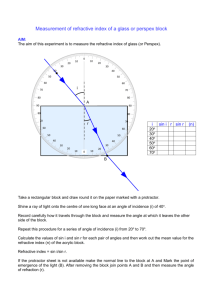
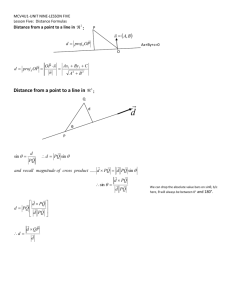
Home | EAT Notes Viscosity Fluids with high viscosity (e.g. honey, treacle, etc) flow slowly through pipes, out of containers, etc. When a body moves through a very viscous fluid there is a large viscous drag force. The reverse is true for fluids with low viscosity (e.g. water, etc). The coefficient of viscosity of a fluid tells us how viscous it is. can be related to the viscous drag F on a sphere of radius r travelling at constant velocity v through the fluid, as shown below: The formula that relates the variables is called Stokes' Law: F = 6rv The units of are Nsm-2. e.g. viscosity of water ~ 1.0 x 10-3 Nsm-2 viscosity of air ~ 1.8 x 10-5 Nsm-2 The viscosities of most fluids are very sensitive to temperature: goes down as temperature goes up Terminal Velocity Consider a ball-bearing falling through a liquid. It has 3 forces acting on it, as shown below: The weight W is a constant force. It is given by: W = mg = density of steel x volume of sphere x g = 4/3r3steelg density of steel where steel = The upthrust U is also a constant force. According to Archimedes' Principle: 4/ U = weight of fluid displaced = density of fluid x volume of sphere x g = 3 where fluid = density of fluid fluidg 3r The viscous drag F, however, increases as the velocity of the sphere increases. According to Stokes' Law: F = 6rv Assume the ball starts from rest at the surface of the fluid - so initially the viscous drag F = 0. Thus, provided W > U, the ball will accelerate downwards to begin with since there is a net downwards force. However, as the ball accelerates, v increases so the viscous drag F increases ..... until eventually ..... U + F becomes equal to W At this point there is no net force on the ball - so it no longer accelerates. It has reached terminal velocity. Substituting all the above expressions into ...... U+F=W (which is true at terminal velocity) we get ..... 4/ 3 3r fluidg + 6rv = 4/3r3steelg An experiment can be done to measure the terminal velocity v, and the above equation can then be re-arranged to find . Streamlined and Turbulent Flow In streamlined or laminar flow the fluid does not make any abrupt changes in direction or speed. It may be thought of as layered flow. In turbulent flow there is chaos, mixing, eddies, etc. Both cases are illustrated below: Turbulence often sets in at higher flow rates or if the fluid has to flow around a shape that is not "aerodynamic" (e.g. a block etc.). It is normally undesirable because it heats the fluid and is therefore inefficient. Properties of Materials Materials deform (i.e. extend or compress) when forces are applied to them. In elastic deformation the material returns to its original dimensions when the the force is removed. In plastic deformation the material remains stretched or compressed when the force is removed. Most materials are elastic for small loads (forces) - but behave plastically if the force is increased beyond a certain point. This is illustrated by the graph below, which is for a typical material (e.g. copper wire): If the load is removed in the elastic region (0 - A), the material returns to 0 (zero extension/compression). If the load is removed in the plastic region (between A and B), the material returns to C. i.e. It is permanently deformed. Ductile materials can be drawn out into long thin shapes (or wires) - e.g. chewing gum. Malleable materials can be deformed (hammered) into a flat shape - e.g. fudge. Ductility and malleability are both examples of plastic behaviour. Brittle materials crack and shatter easily. They are usually stiff, and break before becoming plastic - e.g. glass. Tough materials are not brittle. They can withstand dynamic loads (shocks). They also deform plastically (and absorb a lot of energy) before breaking, Tough materials in the engineering sense are usually stiff as well - e.g. Kevlar. Stiff materials (e.g. steel) need a large force to produce a small deformation. i.e. Their stiffness k is large (see HFS notes). Hard materials are resistant to scratching and indentation - e.g. diamond. Refractometry Refractometry can be used to determine the concentrations of sugar solutions by measuring their refractive index. The diagram below shows a method for measuring the refractive index of a small amount of a liquid - such as a sugar solution: With the eye at A the mark on the glass (X) is not visible, because the light reaching the eye has been totally internally reflected at X - and therefore comes from a point D (not from the mark itself). With the eye at B the mark is just visible because the ray from X to the eye is at the critical angle (C) to the normal at X. With the eye lower than B the mark is easily visible because the light has come from the far side of the glass-liquid boundary. The position B is found and marked with pins. The angles C, r and i are measured. The critical angle C is for the liquid-glass interface. lg = sin 90 / sin C = 1 / sin C index Also: ..... where lg is the liquid to glass refractive ag = sin i / sin r ..... where ag is the air to glass refractive index and ..... ag = al x lg We require al al ..... the refractive index of the liquid = ag / lg al = (sin i / sin r) x sin C = (sin i / sin r) x cos r = sin i / tan r The refractive index of known concentrations of sugar solution can be measured and a graph plotted, as shown below: The sugar concentration of an unknown solution can then be found by measuring its refractive index and using the above graph. Polarisation Light is part of the electromagnetic spectrum. That is to say, it is a transverse wave consisting of sinusoidally varying electric and magnetic fields at right angles to each other. It is "normally" unpolarised. In other words, the oscillations (of the electric and magnetic fields) are in every plane at right angles to the direction of travel - as shown below: In plane polarised light, the oscillations are confined to one plane - as shown below: (Strictly speaking, the above wave just represents the variation of the electric field so there should be another wave at right angles to it representing the variation of the magnetic field. However, it is usual to omit the magnetic wave for the sake of clarity.) Unpolarised light can be polarised by passing it through a polarising filter (such as "Polaroid"). Light reflected off glass, water and many other surfaces is also partially plane polarised. Polarimetry Certain materials (sugar solution being an example) rotate the plane of polarisation of light. In the case of sugar solution, the higher the concentration the greater is the angle of rotation. This fact can be used to measure the strength of a sugar solution using a polarimeter, as shown below: The cylinder is filled with pure water to begin with, and the top polarising filter is rotated until the plane of light that it allows through is at right angles to that allowed through by the bottom filter - i.e. until it blocks the polarised light produced by the bottom filter. The cylinder is then filled to a certain depth with the sugar solution. Since the sugar solution rotates the plane of polarisation, the light is no longer completely blocked, and it is necessary to rotate the top filter in order the block the light again. The angle through which the top filter has to be rotated is the angle through which the plane of polarisation has been rotated. A graph of "angle of rotation" against "sugar concentration" can thus be plotted, as shown below: (N.B. You have to use the same depth of sugar solution each time because the angle of rotation also depends on the depth.) The rotation angle of an unkown solution can be measured, and the sugar concentration read off the graph.