AP Notes Chapter 19
advertisement
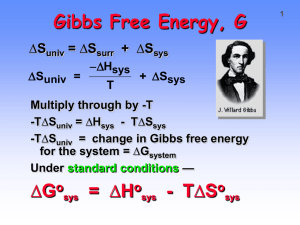
AP Chemistry Chapter 19 Notes CHEMICAL THERMODYNAMICS REVIEW 1st Law of Thermodynamics Energy in the universe is conserved REVIEW Path Function value depends on how process takes place (i.e. q, w) heat q = mCpT REVIEW State Function value independent of path - is a defined reference or zero point (i.e. H) Enthalpy - H zero point 0 Hf of elements in natural form at 0 25 C Enthalpy - H types: Hrxn = nHf 0 nHf (react) 0(prod) Enthalpy - H types: Hfus , Hvap ENTROPY “S” a measure of randomness or disorder of a system Entropy is NOT conserved. The universe seeks maximum disorder. 2nd Law of Thermodynamics for a spontaneous process, entropy increases Entropy - state function zero reference: S = 0 for a perfect crystal at absolute zero Ssolid < Sliquid << Sgas 0 S = standard entropy of elements & cmpds 0 at P = 1 atm; T = 25 C units = J/K mol [Appendix L, text] Calculate S using Hess’ Law S(rxn) = 0 nS (prod) 0 nS (react) Example: Ca(s) + C(gr) + 3/2 O2 CaCO3(s) Suniv = Ssys + Ssurr if Suniv > 0 process spontaneous Suniv = Ssys + Ssurr if Suniv < 0 process ? Suniv = Ssys + Ssurr if Suniv = 0 process ? to relate S and H consider H2O(s) H2O(l) where the water is the system & everything else is the surroundings Temperature Dependence S = J/mol (1/T) Ssurr = -H/T Ssys Ssurr Suniv + + spon Ssys Ssurr Suniv + + - - + spon Y Ssys Ssurr Suniv spon + + + Y - - - N + - Ssys Ssurr Suniv spon + + + Y - - - N + - ? D - + Ssys Ssurr Suniv spon + + + Y - - - N + - ? D - + ? D For a phase change: S 0 phase change Hphase change Tphase change GIBB’S FREE ENERGY G most abstract of thermodynamic state functions w = reversible w1 work P w2 V Definition: G = w + PV w: reversible work PV: pressure/volume work isothermal, reversible path G = w + PV + VP at constant P P = 0 so VP = 0 G = w + PV G = w + PV + VP at constant P G = w + PV at constant V V = 0 so PV = 0 G = w = useful work G cannot be measured must measure G over a process ZERO REFERENCE G = 0 for elements in stable form under Standard Thermodynamic Conditions o T = 25 C ; P = 1 atm Gf = standard Free Energy of Formation from the elements 0 Appendix L, text G follows Hess’ Law: 0 G (rxn) = 0 0 nGf (p) - nGf (r) Summary of Laws of Thermodynamics Zeroth Law: Heat Gain = Heat Loss Summary of Laws of Thermodynamics First Law: Law of Conservation of Energy Summary of Laws of Thermodynamics Second Law: Defines Entropy Summary of Laws of Thermodynamics Third Law: Defines Absolute Zero GIBB’S HELMHOLTZ EQUATION combine G T H G = -aT + H G, H are state functions, thus “a” must be a state function G = -S(T) + H Gibb’s Helmholtz Equation G = H - TS Units on the State Functions kJ H mol J S K mol kJ G mol G H TS G H S T T but H S surr T G Ssurr S T but S surr S S univ G S univ T thus, a process is spontaneous if and only if G is negative Spontaneity controlled by enthalpy (minimum energy) Sponaneity controlled by enthalpy entropy (maximum disorder) Sponaneity controlled by enthalpy entropy both Predict Spontaneity IF H(-) and S(+) G = -H - T(+S) G < 0, => spontaneous Predict Spontaneity IF H(+) and S(-) G = +H - T(-S) G > 0, => NOT spontan Summary of Spontaneity H + + - S + + - G Spont. yes + no + or ? + or ? Uses of the Gibb’s Helmholtz Equation 1. Find the molar entropy of formation for ammonia. 2. Elemental boron, in thin fibers, can be made from a boron halide: BCl3(g) + 3/2 H2(g) ->B(s) + 3HCl(g) Calculate: 0 0 0 H , S and G . Spontaneous? Driving force? 3. Using thermodynamic information, determine the boiling point of bromine. Thermodynamic Definition of Equilibrium Geq = 0 by definition G = H - TS & H = E + PV thus, G = E + PV - TS take derivative of both sides dG = dE + PdV + VdP - TdS - SdT for a reversible process TdS = q derivative used for state function while partial derivative used for path function if the only work is PV work of expansion PdV = w First Law of Thermodynamics E=q-w dE = q - w = 0 thus q = w or TdS = PdV by substitution dG = 0 + PdV + VdP - PdV - SdT or dG = VdP - SdT let’s assume we have a gaseous system at equilibrium, therefore, examine Kp at that constant temperature at constant T SdT = 0 thus dG = VdP G2 P2 G1 P1 dG V dP but nRT V P G2 P2 dP dG nRT G P P 1 1 G2 P2 dP dG nRT G P P 1 1 P2 G2 G1 nRT ln P1 P1 G1 G2 RT ln P2 n if “condition 2” is at standard thermodynamic conditions, then 0 G2 = G and P2 = 1 atm thus G G RT ln P 0 n determine G for aA + bB cC + dD where all are gases G = Gprod - Greact = cGC + dGD - aGA - bGB but cG C cG RT ln P 0 C and likewise for the others c and cG dG aG bG 0 C 0 D G 0 rxn 0 A 0 B G G (PC ) (PD ) RT ln a b (PA ) (PB ) c 0 rxn but (PC ) (PD ) ? (P )a (P )b A B c d d Thus, G = 0 G + (RT) ln Q But aA + bB cC + dD G = 0 thus G 0 rxn RT ln K P or in general G 0 rxn RT ln K eq THERMODYNAMICS & EQUILIBRIUM 3. Using thermodynamic information, determine the boiling point of bromine. Thermodynamics and Keq FACT: Product-favored systems have Keq > 1. Thermodynamics and Keq Therefore, both ∆G˚rxn and Keq are related to reaction favorability. Thermodynamics and Keq Keq is related to reaction favorability and thus to ∆Gorxn. The larger the value of K the more negative the value of ∆Gorxn Thermodynamics and Keq o ∆G rxn= - RT lnK where R = 8.314 J/K•mol Thermodynamics and Keq ∆Gorxn = - RT lnK Calculate K for the reaction N2O4 2 NO2 ∆Gorxn = +4.8 kJ K = 0.14 When ∆G0rxn > 0, then K < 1 ∆G, ∆G˚, and Keq ∆G is change in free energy at non-standard conditions. ∆G is related to ∆G˚ ∆G = ∆G˚ + RT ln Q where Q = reaction quotient ∆G, ∆G˚, and Keq When Q < K or Q > K, reaction is spontaneous. When Q = K reaction is at equilibrium When ∆G = 0 reaction is at equilibrium Therefore, ∆G˚ = - RT ln K ∆G, ∆G˚, and Keq Product favored reaction –∆Go and K > 1 In this case ∆Grxn is < ∆Gorxn , so state with both reactants and products present is MORE STABLE than complete conversion. ∆G, ∆G˚, and Keq Product-favored reaction. 2 NO2 N2O4 ∆Gorxn = – 4.8 kJ Here ∆Grxn is less than ∆Gorxn , so the state with both reactants and products present is more stable than complete conversion. Thermodynamics and Keq Overview o ∆G rxn is the change in free energy when reactants convert COMPLETELY to products. Keq is related to reaction favorability. When ∆Gorxn < 0, reaction moves energetically “downhill” 4. For the following reaction, calculate the temperature at which the reactants are favored. 1 3 N2 (g) H2 (g) NH3 (g) 2 2 THERMODYNAMICS OF CHEMICAL REACTIONS 5. How much useful work can be obtained from an engine fueled with 75.0 L of hydrogen at 10 C at 25 atm? 6. The reaction to split water into hydrogen and oxygen can be promoted by first reacting silver with water. 2 Ag(s) + H2O(g) Ag2O(s) + H2(g) Ag2O(s) 2 Ag(s) + 1/2 O2(g) 0 H , 0 S Calculate 0 and G for each reaction. Combine the reactions 0 and calculate H and 0 G for the combination. Is the combination spontaneous? At what temperature does the second reaction become spontaneous? 7. The conversion of coal into hydrogen for fuel is: C(s) + H2O(g) CO(g) + H2(g) Calculate 0 G and Kp at 0 25 C. Is the reaction spontaneous? At what temperature does the reaction become spontaneous? Calculate the temperature at which -4 K = 1.0 x 10 . 8. The generation of nitric acid in the upper atmosphere might destroy the ozone layer by: NO(g) + O3(g) NO2(g) + O2(g) 0 G Calculate (reaction) 0 and K at 25 C.