CH 2
advertisement

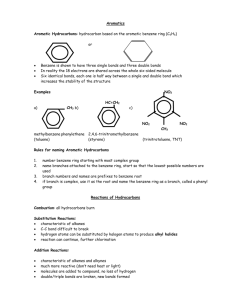
Organic Chemistry Unit 10 What is Organic chemistry? • What does organic mean to you? • The name organic was given to molecules found in living organisms • Now, organic chemistry refers to the chemistry of carbon compounds – Carbon is important to life because of its ability to form an endless number of molecules • CH4 – methane gas • Proteins • Cotton, wool, silk • CH3CH2OH - Ethanol Organic Compounds Typically, organic compounds Contain carbon. Have covalent bonds. Have low melting points. Have low boiling points. Are flammable. Are soluble in nonpolar solvents. Bonding in Organic Compounds • Carbon has 4 valence electrons (lone electrons) • • C• • • This means there are four places for it to bound to other atoms in order for carbon to achieve an octet •4 1• • C • 3 2 Bonding with Hydrogen Carbon has 4 lone electrons; hydrogen has 1. • • C• H• • To achieve an octet, carbon forms four bonds. H H •• | HCH H—C—H CH4 , methane •• | H H Tetrahedral Nature of Carbon • When carbon forms four bonds to other atoms, the bonds are situated 109.5o apart from each other. • This arrangement is a tetrahedral arrangement. Organic Molecules • In organic molecules, valence electrons form covalent bonds between carbon atoms • Molecules consisting of only carbon and hydrogen are call hydrocarbons H •• HC •• H H •• CH •• H H H | | H—C—C—H | | H H Ethane,CH3CH3 Other Elements • Carbon in organic compounds also commonly forms covalent bonds with N, O, S, and halogens (Cl, Br) Learning Check • Complete the structure of the organic molecule by adding the correct number of hydrogen atoms. C—C—C Learning Check • Complete the structure of the organic molecule by adding the correct number of hydrogen atoms. H H H | | | H—C—C—C—H | | | H H H Alkanes • Hydrocarbons that contain only carbon-carbon single bonds • General formula = CnH2n+2 – n = number of carbons Uses of Alkanes • Small number of carbons (1 – 4 carbons) – gases – Heating fuels – propane, butane • 5 – 8 carbons – Liquids – Fuels – gasoline, kerosene, diesel, jet fuel • 18 + carbons – Waxy solids – Waxes (paraffins), Vaseline Conformation of Alkanes • Because of the tetrahedral shape of carbon bonds, carbon bonds are in a zigzag pattern • Atoms can rotate around a single carbon-carbon bond – Different arrangements that can occur because of this are called conformations Expanded and Condensed Formulas • Expanded structural formula = all individual bonds (indicated with dashes) and atoms are drawn • Condensed structural formula =each carbon atom is grouped with its bonded hydrogen atoms. Subscripts are used to indicate number of H’s and bonds are indicated with dashes • Skeletal Formula= only carbons and bonds(as dashes) are represented- Hydrogens are implied Expanded and Condensed Formulas • Line bond formulas = lines represent carbon-carbon bonds. No individual atom is indicated. Hydrogens and Carbons are implied. • Molecular Formula= Atoms are represented and subscripts are used to indicate the number of each atom. No bonds are drawn. • Geometric Formula= Similar to line bond formulas, but used for cyclic compounds. IUPAC • International Union of Pure and Applied Chemistry • Determined protocol for naming organic compounds • Pentane – Prefix states number of carbons • Pent - = five carbons – Suffix shows kind of compound • -ane = alkane, only single carbon bonds Prefixes (Table 11.2) Cycloalkanes • Hydrocarbons do not need to be in a chain, they can also form circular structures Cycloalkanes • Cycloalkanes: • Are rings of carbons that can be drawn as geometric figures. • Have a general formula of CnH2n or 2 H less than the alkane. • propane C3H8 cyclopropane C3H6. • butane C4H10 cyclobutane C4H8. • Are named with the prefix cyclo- in front of the corresponding alkane name. Table 11.4 LINE BOND FORMULAS FOR SOME CYCLOALKANES Cyclopropane Cyclobutane Cyclopentane Cyclohexane C3H6 C4H8 C5H10 C6H12 Naming Alkanes • Molecular formulas do not tell us the structure – constitutional isomers • Carbon compounds can be continuous-chain or branched-chained Substituents • Substituents are groups of atoms that replace a hydrogen on a carbon chain – Blue flashcards! *need to know!!* • If the substituent is a hydrocarbon, it is called an alkyl group – The alkyl group is named by replacing the – ane with -yl Some of these included in Table 11.5 Steps for naming alkanes 1. Name the longest continuous chain of carbons as the main chain 2. Number the carbon atoms in the main chain starting on the end nearest a substituent -Where there are 2 or more substituents, the main chain should be numbered to give the lowest possible number set Naming Alkanes Cont. 3. Give the location and name of each alkyl group in front of the name of the main chain - use prefixes (di-, tri-) if a group appears more than once 4. List the substituents in alphabetical order Naming CH3-CH-CH2-CH-CH2-CH3 CH3 CH2CH3 • 1. Name Longest Chain First= hexane • 2. Number Carbons from the end with the nearest substituent. 1 2 3 4 5 6 CH3-CH-CH2-CH-CH2-CH3 CH3 CH2CH3 3. Give the location and name of each alkyl group in front of the name of the main chain 2-methyl-4-ethylhexane 4. List substituents in alphabetical order. 4-ethyl-2-methylhexane Naming CH3-CH-CH2-CH2-CH-CH3 CH3 CH2CH3 • 1. Name Longest Chain First= heptane • 2. Number Carbons from the end with the nearest substituent. 1 2 3 4 5 CH3-CH-CH2-CH2-CH-CH3 CH3 6 CH2CH3 7 3. Give the location and name of each alkyl group in front of the name of the main chain 2,5-dimethylheptane 4. List substituents in alphabetical order. Fine as is! Try a Few! • On board exercise! Drawing Structural Formulas Step 1 – Draw the main chain of carbon atoms Step 2 – Draw the substituents on the main chain in the positions indicated by the location numbers Step 3 – Fill in the correct number of hydrogen atoms to give four bonds to each carbon atom Draw 2,3-dimethylpentane 1. Draw Main Chain of Carbon atoms C-C-C-C-C 2. Draw the substituents on the main chain in the positions indicated by the location numbers C-C-C-C-C C C 3. Fill in the correct number of hydrogen atoms to give four bonds to each carbon atom CH3-CH-CH-CH2-CH3 CH3 CH3 Try a few! • 2,3,5-trimethylhexane • 3-ethylpentane • 4-isopropyloctane Isomers • Molecules with the same molecular formula but different structural formula • Example: C5H12 CH3-CH2-CH2-CH2-CH3 CH3-CH-CH2-CH3 C H3 C H3 CH3-C-CH3 C H3 Constitutional Isomers • Most organic compounds have structural isomers and their number increases as the number of atoms increases Physical Properties of Constitutional Isomers Different structural arrangement can result in very different physical properties Drawing Isomers • Step 1 – Draw the longest continuous chain • Step 2 – Remove one carbon from the chain and attach it as a methyl group in as many locations as possible • Step 3 – Remove another carbon atom from the main chain and attach as another alkyl group Try Some • Draw isomers for C4H10 – Practice Naming!! Haloalkanes • An alkane in which halogen atoms replace one or more hydrogens • Used as solvents and anesthetics • CFC’s (chlorofluorocarbons) were used a propellants in aerosols – React with ozone in the upper atmosphere – Resulted in ozone depletion over the Antarctic Naming Haloalkanes • IUPAC names for halogen substituents are: • • • • Fluorine = fluoroChlorine = chloroBromine = bromoIodine = iodo- • The halo-substituents are numbered and arranged alphabetically, like we did before Other substituents • Use the same naming rules – Number position on the parent chain – Put in alphabetical order CH3-CH-CH2-CH3 Cl CH3-CH-CH-CH2-CH3 Cl Br Naming Cycloalkanes A cycloalkane with: One substituent is named by placing the name of the substituent in front of the cycloalkane name. Two or more substituents is named by numbering the ring in the direction that gives the lower numbers to the substituents. Cycloalkanes with Side Groups CH3 methylcyclopentane CH3 CH3 1,2-dimethylcyclopentane CH3 CH3 1,2,4-trimethylcyclohexane CH3 Try Some! • On Board Exercise Worksheet 1 • You can now complete worksheet 1. Chemical Properties of Alkanes Alkanes are typically not very reactive due to strong C-C single bonds. The most typical reaction is combustion, where an alkane reacts with oxygen to produce carbon dioxide, water, and energy. alkane + O2 CO2 + H2O + energy Combustion A fuel such as propane reacts with oxygen and burns, producing CO2 and H2O. Propane is burned to obtain energy and heat for cooking or warming a room. C3H8 + 5O2 3CO2 + 4H2O Incomplete combustion • It is dangerous to burn fuels in a closed room • With limited amounts of oxygen, incomplete combustion occurs – This produced carbon monoxide, which is a toxic gas 2CH4(g) + 3O2(g) 2CO(g) + 4H2O(g) + heat Classifying Carbon Atoms • Primary Carbons (1o)- Bonded to only one other carbon atom • Secondary Carbons (2o)- Bonded to two other carbon atoms • Tertiary Carbons (3o)- Bonded to three other carbons Functional Groups • Millions of compounds possible • However, certain structures behave in similar manners • These are called functional groups • For now: • Alkanes – Saturated Hydrocarbons • Alkenes, Alkynes, Aromatics – Unsaturated Hydrocarbons Background: Double Bonds • Two nonmetal atoms can share more than one set of electrons • Sharing four electrons = double bond H H H H •• •• •• • •C • • •C• Background: Triple Bonds • Two nonmetals can also form triple bonds = sharing 6 electrons Alkenes, Alkynes and Aromatics • Alkanes contain only single bonds • Alkenes contain one or more double bonds • Alkynes contain one or more triple bonds • Aromatic ring (benzene) is 6 carbons cyclized with alternating double bonds Ch. 12-Unsaturated Hydrocarbons Unsaturated hydrocarbons: Have fewer hydrogen atoms attached to the carbon chain than alkanes. Are alkenes with double bonds or alkynes with triple bonds. Alkenes • Contain at least one double bonds • Important in manufacturing and in human and plant functioning – Hormones – Ripening fruit – Ethene used to make polymers Naming Alkenes and Alkynes In the IUPAC system, the –ane ending of the corresponding alkane is changed to –ene for alkenes and to –yne for alkynes. Naming Alkenes and Alkynes When the carbon chain has 4 or more C atoms, the chain is numbered to give the lowest number to the double or triple bond. 1 CH2=CH—CH2—CH3 1-butene 2 CH3—CH=CH—CH2—CH3 2-pentene 3 CH3—CH2—CC—CH2—CH3 3 -hexyne More Naming • If you have more than 1 double or triple bond: – Name them as dienes or diynes • CH2=CH—CH2—CH2=CH3 1 2 3 4 1, 4 - pentadiene 5 • CHC—CH2—CC—CH2—CH3 ???? More Naming • If you have more than 1 double or triple bond: – Name them as dienes or diynes • CH2=CH—CH2—CH2=CH3 1 2 3 4 1, 4 - pentadiene 5 • CHC—CH2—CC—CH2—CH3 1, 4 - heptadiyne Alkenes and Alkynes with Substituents • 1. Name the longest carbon chain that contains the double or triple bond • 2. Number the chain starting at the end nearest the double or triple bond. 4-methyl-1-pentene Try One! Naming Cyclic Alkenes and Alkynes • Use –ene instead of -ane • The double bond of a cycloalkene is understood to be between Carbon 1 and Carbon 2. Number from there to get the lowest possible number set. Practice! • On Board Exercise Cis-Trans Isomers Double Bonds do not rotate freely! Introducing a new type of isomer! Cis-Trans Isomers Two isomers are possible when groups are attached to the double bond. In a cis isomer, groups are attached on the same side of the double bond. symmetrical In the trans isomer, the groups are attached on opposite sides. unsymmetrical Naming Cis-Trans Isomers The prefixes cis or trans are placed in front of the alkene name when there are cis-trans isomers. Br Br C C H H Br C C H cis-1,2-dibromoethene H Br trans-1,2-dibromoethene Worksheet 2 • You can now complete worksheet 2. Addition Reactions Double and triple bonds are weaker than single bonds More reactive In the addition reaction, reactants are added to the carbon atoms in the double or triple bond. Addition Reactions • H2C=CH2 + A—B H2C—CH2 A B Hydrogenation In hydrogenation, hydrogen atoms add to the carbon atoms of a double bond or triple bond. A catalyst such as Pt or Ni is used to speed up the reaction. H H H2C CH2 + H2 Pt H2C CH2 H H HC CH + 2H2 Ni HC CH H H Hydrogenation of Oils When hydrogen adds to the double bonds in vegetable oils, the products are solids at room temperature. Halogenation • In halogenation, halogen atoms add to the carbon atoms of a double bond or triple bond. Br Br H2C CH2 + Br2 H2C CH2 Cl Cl HC C CH3 + 2Cl2 H C C CH3 Cl Cl Testing for Double and Triple Bonds • When bromine (Br2) is added to an alkane, the red color of bromine persists. • When bromine (Br2) is added to an alkene or alkyne, the red color of bromine disappears immediately. Hydrohalogenation • In hydrohalogenation, the atoms of a hydrogen halide add to the carbon atoms of a double bond or triple bond. H CH3 CH CH CH3 + HCl + HBr Cl CH3 CH CH CH3 H Br Markovnikov’s Rule • When an unsymmetrical alkene undergoes hydrohalogenation, the H in HX adds to the carbon in the double bond that has the greater number of H. H Cl CH3 CH CH2 Does not form CH3 CH CH2 + HCl C with the most H Cl H CH3 CH CH2 Product that forms Hydration Adds Water • In hydration, H and OH from water add to the carbon atoms of a double bond or triple bond to form alcohols (OH). • The reaction is catalyzed by acid H+. + H CH3 CH CH2 + HOH CH3 CH CH2 H+ + HOH OH H H OH Worksheet 3 • You can now complete worksheet 3. Aromatic Compounds Benzene is An aromatic compound. A ring of 6 C atoms and 6 H atoms. Alternating single and double bonds Aromatic compounds often have fragrant odors Aromatic Compounds in Nature and Medicine Naming Aromatic Compounds A benzene with a single substituent is often named as a benzene derivative. CH3 Cl Methylbenzene Chlorobenzene A benzene ring as a substituent is called a phenylgroup Some Common Names Some substituted benzene rings have common names that have been in use for many years. CH3 Toluene (Methylbenzene) NH2 Aniline (Benzenamine) Also, know naphthalene (2 fused rings) OH Phenol (Hydroxybenzene) Naming Aromatic Compounds A benzene ring with two or more substituents is numbered to give the lowest numbers to the side groups. Common names use the prefixes ortho- (1,2-), meta- (1,3-) and para- (1,4-). CH3 Br Cl Br Cl 1,2-dimethylbenzene (o-dibromobenzene) 1,3-dichlorobenzene (m-dichlorobenzene) Cl 4-chloromethylbenzene (p-chlorotoluene)