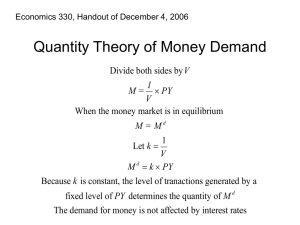
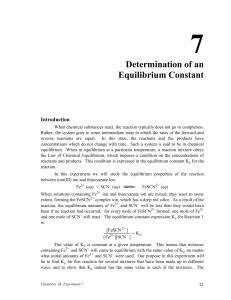
Equilibrium-Intro
advertisement

Equilibrium Constant • Turn in CV Final Report and Pre-lab in folders • Drop your Group Form • Download example spreadsheet from Dr. Ng’s website • Today – look at a dynamic equilibrium reaction between varying concentrations of reactants for the following equation: Fe3+(aq) + clear SCN-(aq) clear FeSCN2+(aq) ORANGE To determine the equilibrium constant for this reaction: [ FeSCN 2 ] K [ Fe3 ][ SCN ] Equilibrium Constant Step One: Prepare the 8 solutions (7 reactions plus blank) on bottom of pg. 5 use one exclusive pipette per reactant one for Fe+3 (Same as Fe(NO3)3) or [Fe(H2O)6]+3 one for SCN- (Same as KSCN) one for DI water Take enough of each solution to do mixing at your benchtop (how much do you need?) use medium or large test tubes *DO NOT USE SMALL** LABEL YOUR SOLUTIONS!!!! Equilibrium Constant Step Two: Calibrate the instrument ( = 447 nm ) (similar to last week) 0%T is door shut, no cuvet 100 %T is the prepared BLANK solution (bottom of page 5) Step Three: Record % T for each solution (not absorbance, page 3, 4, 6, 7) Use only one cuvet No need to clean cuvet between solutions – simply dump cuvet contents in a beaker from your drawer, and rinse with a little of the next solution, then fill ¾ full with that solution ALL WASTE IN WASTE BEAKER IN FUME HOOD!!!!!!! Equilibrium Constant Step Four, WHAT TO DO WITH THE DATA: Make a Calibration Curve using given data on page 6 and an excel spreadsheet • y vs. x (Absorbance vs. concentration) • From the calibration curve, determine linear equation • Linear equation will be in the form • y = mx + b • Abs = m*[Fe(SCN)]+2 + b (b is y intercept and should be 0 abs at 0 concentration) • Abs = [·ℓ] * c Beer’s Law • Abs = [·ℓ] * [Fe(SCN)]+2 • [·ℓ] = m • Get [·ℓ] => from equation on Calibration Curve Equilibrium Constant Calibration Curve for FeSCN2+ 0.900 0.800 Absorbance 0.700 ε=5126.7 units!!! 0.600 0.500 0.400 Abs = 5126.7*C - 0.0028 R2 = 0.9998 0.300 0.200 0.100 0.000 0.0E+00 5.0E-05 1.0E-04 1.5E-04 2.0E-04 [Fe(SCN)]2+ (M) Made from data on page 6, remember slope = ε * l, and l = 1 cm Use information from linear equation of the calibration curve to convert absorbance values into c=[FeSCN2+] for your 7 solutions! Equilibrium Constant – Data Table (make in EXCEL) Using Dr. Ng’s example, make a similar table in EXCEL and input YOUR data for your 7 solutions: Equilibrium Constant – LAB REPORT Title Page Purpose Results Calibration Curve with ε clearly marked on chart – full page and landscaped excel data spreadsheet (formatted similar to example .pdf file with gridlines and data) sample calcs for each column of spreadsheet (choose one row) significant figures and units Conclusion – purpose, errors, future suggestions, TWO post-lab questions (see page 8 of laboratory procedure) Individual Checked-Pre-labs