Data Management
advertisement

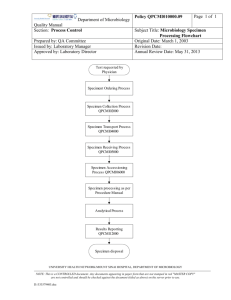
HIV Drug Resistance Training Module 12: Data Management 1 A Systems Approach to Laboratory Quality Organization Personnel Equipment Stock Management Quality Control Data Management SOPs, Documents & Records Occurrence Management Assessment Process Improvement Specimen Management Safety & Waste Management 2 Data Management Information flow Data collection & management Patient privacy & confidentiality Computer skills 3 Topics Importance of Data Management Components of Data Management Policies and Procedures 4 Objectives At the end of this module, you will be able to: Describe the importance of data management. Describe what data needs to be recorded and organized prior to, during, and after the assay. Identify policies and procedures needed to support efficient collection and retrieval of data. 5 importance of data management Why is data management important? 6 Data Management Means… An organized way to record, store and retrieve data associated with pre-testing, in process testing, and post-testing information Associated laboratory procedures and policies instructing operators and supervisors on how to use available systems and tools 7 Why Data Management is Critical Ensure high quality results Enable detailed tracking and troubleshooting Retrieval and reconstruction of assay-associated information Reproduction of results at a later date Ongoing laboratory and personnel QAI 8 components of data management What is involved in data management? In what parts of the process is it especially important to collect data? How can we ensure that the data we collect is organized so we can find it when we need it? 9 Small Group Brainstorming Discuss! Think of the process from receiving a sample to discarding it. – What data would you want to gather specific to that sample? Why? How? – What aggregate data would you want to capture? Why? How? Data We Need Why? How? 10 Data Management Involves… Before Testing During Testing After Testing Other Uses • Accessioning information • Equipment maintenance data (e.g., calibration results) • Critical reagent lot numbers, expiration dates • Operator information • In-process results such as PCR product (quantitative or qualitative) • internal QC control results • Individual sample and positive control final sequences • Raw chromatogram data • Final reports • Phylogeny • Subtyping 11 Prior to Testing Accessioning information – Specimen ID (unique) – Patient information (name, DOB, other ID, clinical parameters) – Specimen type, date/time of collection, storage/shipping conditions – Receipt date/time, condition Equipment maintenance – Dates of major service and/or calibration – Calibration/QC results 12 During Testing Critical reagents – Lot numbers, expiration dates Operator information (who did what) In-process results – – – – PCR product (quantitative or qualitative) Sequencing signal intensity Extent of manual sequence editing Date/time of completion of intermediate steps Internal QC control results – Positive and negative plasma controls – Positive and negative PCR controls – Positive control for sequencing 13 After Testing Final nucleotide sequences – Individual samples – Positive control(s) Raw chromatogram data Final reports – Interpretation system and version – Date/time reported 14 Other Uses Phylogeny Subtyping 15 Policies and Procedures What policies do we need to develop or enhance to ensure the quality control of data gathered for genotyping? 16 Policies: Manual Processes Policies for what data to record Standardized forms for collection of data Controlled document, like SOPs Policy for organization, indexing and storage to facilitate retrieval Training 17 Policies: Automated Processes Policies for what data to record SOPs for operation of software for: – Data entry – QA checks/verification – Archival of primary results (e.g. agarose gel images, raw chromatogram data etc.) – Report generation Training 18 Policies: Gathering Data Information generated/recorded by – Lab technicians performing the assays – Supervisors Link all information to its sample via unique accession ID 19 Policies: Storing Data Safe and reliable location – Paper records: dedicated room – Electronic records: regular back-up Protect Patient/participant information – Paper records: restricted access – Electronic records: user-level access directories 20 Policies: Organizing Data for Efficient Retrieval Unique identifier (accession number) on all records – allows for searching and retrieval of all data Electronic systems – Lab information system (LIS) – Databases and spreadsheets Paper records: indexing system 21 Policies: Sharing Data In general, all in-process data are kept internally and not shared outside the lab Results only shared with original client/study lead Protect patient/participant information (except to primary physician) Following publication, or decision not to publish, make sequences publicly available WHO database available for managing data generated during WHO surveys – Version 2 early 2010 22 Discussion Think of your current lab policies related to data management. What changes, if any, should be made to these policies to ensure the quality of genotyping results for policy making at the national level? 23 Procedures Procedures for Data Management may be integrated into testing procedures, or implemented as separate SOPs See handout for an example 24 Reflection What does data management mean? What are the different components of data management in the life cycle of the sample? For aggregating data? What work does your lab need to do in this area? 25 Key Messages Genotyping essentially generates INFORMATION about a patient specimen Managing all the information and data related to each specimen is a crucial responsibility of every lab 26 Summary Importance of Data Management Components of Data Management Policies and Procedures 27