water and life - Tacoma Community College
advertisement

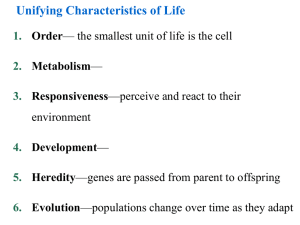
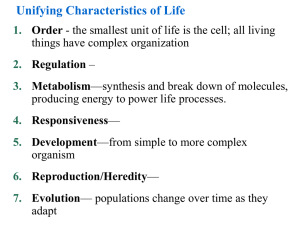
Unifying Characteristics of Life 1. Order— the smallest unit of life is the cell 2. Metabolism— 3. Responsiveness—perceive and react to their environment 4. Development— 5. Heredity—genes are passed from parent to offspring 6. Evolution—populations change over time as they adapt Biological Organization Fig 1.3 4. Cell :The simplest entity that has all the properties of life 3. Organelle : 2. Molecule : 1.Atom:smallest unit of an element that still retains the element’s properties 7. Organ System:A group of body parts that carries out a particular function in an organism 6. Organ : 5. Tissue :A group of similar cells that carries out a particular function in an organism 9. Population: 10. Community: 8. Organism: individual composed of many coordinated organ systems 11. Ecosystem: 12. Biosphere: Those regions of the earth’s waters, crust and atmosphere in which organisms can exist.The global ecosystem Cells and Their DNA • The cell is the simplest structure that can perform all activities required for life • Cell Theory: • There are two major types of cells 1. 2. • All cells use DNA as the chemical material of genes – Genes: The Diversity of Life • The diversity of known life includes 1.7 million species • Estimates of the total diversity range from 5 million to over 30 million species The Unity and Diversity of Life EUKARYOTES Animals Plants Fungi Protists Bacteria PROKARYOTES Universal Ancestor Archaea: Bacteria adapted to extreme environments The Three Domains of Life • The three domains of life are: 1. Bacteria 2. Archaea 3. Eukarya Domain Archaea Domain Bacteria Domain Eukarya Kingdom Protista Kingdom Plantae Kingdom Fungi Kingdom Animalia Fig 1.9 Unity in the Diversity of Life • Underlying the diversity of life is a striking unity, especially at the lower levels of structure • Evolution accounts for this combination of unity and diversity EVOLUTION: BIOLOGY’S UNIFYING THEME • The history of life is a saga of a restless Earth billions of years old – Fossils document this history Fig 1.10 • Life evolves Each species is one twig of a branching tree of life extending back in time Fig 1.11 Ancestral bear • Darwin’s book developed two main points 1. Descent with modification 2. Natural selection Natural Selection • Darwin was struck by the diversity of animals on the Galápagos Islands • He thought of origin of new species and adaptation to the environment the as closely related processes Descent with modification Cactus ground finch Medium ground finch Large ground finch Small ground finch Large cactus ground finch Small tree finch Vegetarian finch Medium Woodpecker tree finch finch Large Mangrove tree finch finch Gray Green warbler warbler finch finch Sharp-beaked ground finch Seed-eaters Cactus-flower Bud-eater -eaters Ground finches Insect-eaters Tree finches Fig 1.13 Common ancestor from South American mainland Warbler finches Darwin’s Conclusion •Darwin synthesized the concept of natural selection from two observations: • Fact 1: • Fact 2: • Conclusion: Unequal reproductive success Fig 1.14: Natural Selection The Evolution of Diversity • Different species have different traits. These arise from: • Mutations – – heritable changes in DNA. Mutations are adaptive if they change the organism’s ability to get food, mate, etc. • Evolution – • Natural selection - adaptive traits tend to increase over time. It is the mechanism of evolution • Darwin’s publication of The Origin of Species fueled an explosion in biological research – Evolution is one of biology’s best demonstrated, most comprehensive, and longest lasting theories – Evolution is the unifying theme of biology BASIC CHEMISTRY • Organisms and all other things in the universe consist of matter • Matter is anything that occupies space and has mass Periodic table of the elements Atomic number Element symbol Mass number • 25 Elements are essential to life • C, H, O, N: 96% of the weight of the human body Fig 2.3 Elements • Elements can combine to form compounds • Compounds • Examples of Compounds: Atom: (a) Hydrogen atom (b) Carbon atom Proton Neutron Atomic nucleus Electron (c) Oxygen atom First shell Second shell Atomic Structure • The subatomic particles of an atom Electron Proton Neutron Nucleus -Consists of neutrons and protons • Elements • Atomic Number: • Mass number sum of the number of protons and neutrons Chemical Properties of Atoms • Electrons • The number of electrons in the outermost shell… First electron shell: can hold 2 electrons Outermost electron shell: can hold 8 electrons Electron Hydrogen (H) Atomic number = 1 Carbon (C) Atomic number = 6 Fig 2.7 Nitrogen (N) Atomic number = 7 Oxygen (O) Atomic number = 8 Chemical Bonding and Molecules • Chemical reactions: – 2 types of molecular bonding: • Ionic Bonds • Covalent bonds Ionic Bonds • When an atom loses or gains electrons, it becomes electrically charged = ion Sodium atom Chlorine atom – Ionic bonds Complete outer shells Na Fig 2.8 Cl Sodium chloride (NaCl) Atoms: electrically neutral Ions: Electrically charged (b) Hydrogen ion (H+) (a) Hydrogen atom (H) 1 electron No electron 1 proton 1 proton No electrical charge (d) Sodium ion (Na+) (c) Sodium atom (Na) 11 electrons 11 protons No electrical charge 10 electrons 11 protons Covalent Bonds Fig 2.9 Covalent bonding in water Oxygen atom with unfilled shell Water molecule (H2O) Full shell with 8 – Slightly electrons negative Covalent bond (shared pair of electrons) + + Full shells with 2 electrons each Hydrogen atoms with unfilled shells Slightly positive WATER AND LIFE • Life on Earth began in water and evolved there for 3 billion years • The abundance of water is a major reason Earth is habitable The Structure of Water • The polarity of water results in….. () () () Hydrogen bond () () () () () Fig 2.10 Water’s Life-Supporting Properties • Hydrogen bonding explains most of water’s lifesupporting properties: 1. Water as the Solvent of Life Ion in solution Fig 2.16 Salt crystal Dissolving of Sodium Chloride, NaCl, in Water Salt Electrical attraction Water molecules dissolve NaCl, breaking ionic bond Water Water molecules (H2O) Hydrogen bonds Edge of one salt crystal Ionic bond 2. Cohesion = Microscopic tubes Fig 2.12 3. Water Moderates Temperature • Because of hydrogen bonding, water has a strong resistance to temperature change • Water can absorb and store large amounts of heat while only changing a few degrees in temperature. • How? The Four Most Important Organic Biological Compounds 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic Acids 1) Carbohydrates • C:H:O ratio is 1:2:1 • Simple sugars: (CH20)n Structural units, used to make larger, storage compounds: 1. Starch – 2. Glycogen – 3. Cellulose – Fig 3.13 Glucose Fructose Monosaccharides C6H12O6 (Simple sugars) Glucose Fructose Formation of a Disaccharide H2O (water) C12H22O11 Sucrose A portion of a polysaccharide 2. Lipids • Non-polar, hydrophobic (don’t dissolve in water) • (CH)nCOOH • Functions: A) Fats • Triglycerides – most abundant lipids in body, abundant energy! Fig. 3.15 B) Phospholipids 3) Proteins Fig. 3.20 Fig 3.21 Proteins continued Primary structure Fig 3.22 Fig 3.24 4) Nucleic Acids • DNA & RNA • Monomers of Nucleotides Fig 3.26 Fig 3.27 The nitrogenous bases of DNA RNA contains: ribose instead of deoxyribose, and uracil instead of thymine Fig 3.29 Fig 3.28: The structure of DNA